ORIGINAL ARTICLE
Composition of fatty acids in hemp leaves (Cannabis sativa L.) under the impact of aphids and a herbicide
1
Faculty of Biology and Nature Protection, University of Rzeszów, Rzeszów, Poland
2
Department of Entomology and Environmental Protection, Poznan University of Life Sciences, Poznań, Poland
3
Faculty of Biotechnology, Collegium Medicum, Rzeszów, Poland
4
Department of Pathogen Genetics and Plant Resistance, Institute of Plant Genetics, Polish Academy of Sciences, Poznań, Poland
These authors had equal contribution to this work
A - Research concept and design; B - Collection and/or assembly of data; C - Data analysis and interpretation; D - Writing the article; E - Critical revision of the article; F - Final approval of article
Submission date: 2024-03-02
Acceptance date: 2025-01-08
Online publication date: 2025-07-01
Corresponding author
Malgorzata Jedryczka
Department of Pathogen Genetics and Plant Resistance, Institute of Plant Genetics, Polish Academy of Sciences, Strzeszynska 34, 60-479, Poznan, Poland
Department of Pathogen Genetics and Plant Resistance, Institute of Plant Genetics, Polish Academy of Sciences, Strzeszynska 34, 60-479, Poznan, Poland
Journal of Plant Protection Research 2025;65(2):241-254
HIGHLIGHTS
- Graminicide quizalofop–P–tefuryl rapidly degraded in hemp plants after treatment.
- Hemp aphids feeding on hemp treated with quizalofop increased their reproduction
- Aphid feeding and herbicide application affected the fatty acid composition in hemp
- Myristic and oleic acids were increased, while linolenic acid was decreased
- We speculate that aphids manipulate with plant defense using jasmonic acid pathway
KEYWORDS
TOPICS
ABSTRACT
Cannabis aphid Phorodon (Diphorodon) cannabis Passerini 1860 is an economically important
pest of oil hemp (Cannabis sativa L.) and is controlled by insecticides. Oil hemp crops
are treated with herbicides, which are non-target pesticides for aphids but may also affect
aphid populations. Such ecological implications of plant protection products are rarely investigated.
The aim of the present research was to better understand plant ‒ aphid ‒ herbicide
interactions, specifically, changes of fatty acids (FAs) in leaves, caused by cannabis
aphids and a common herbicide used in hemp fields.
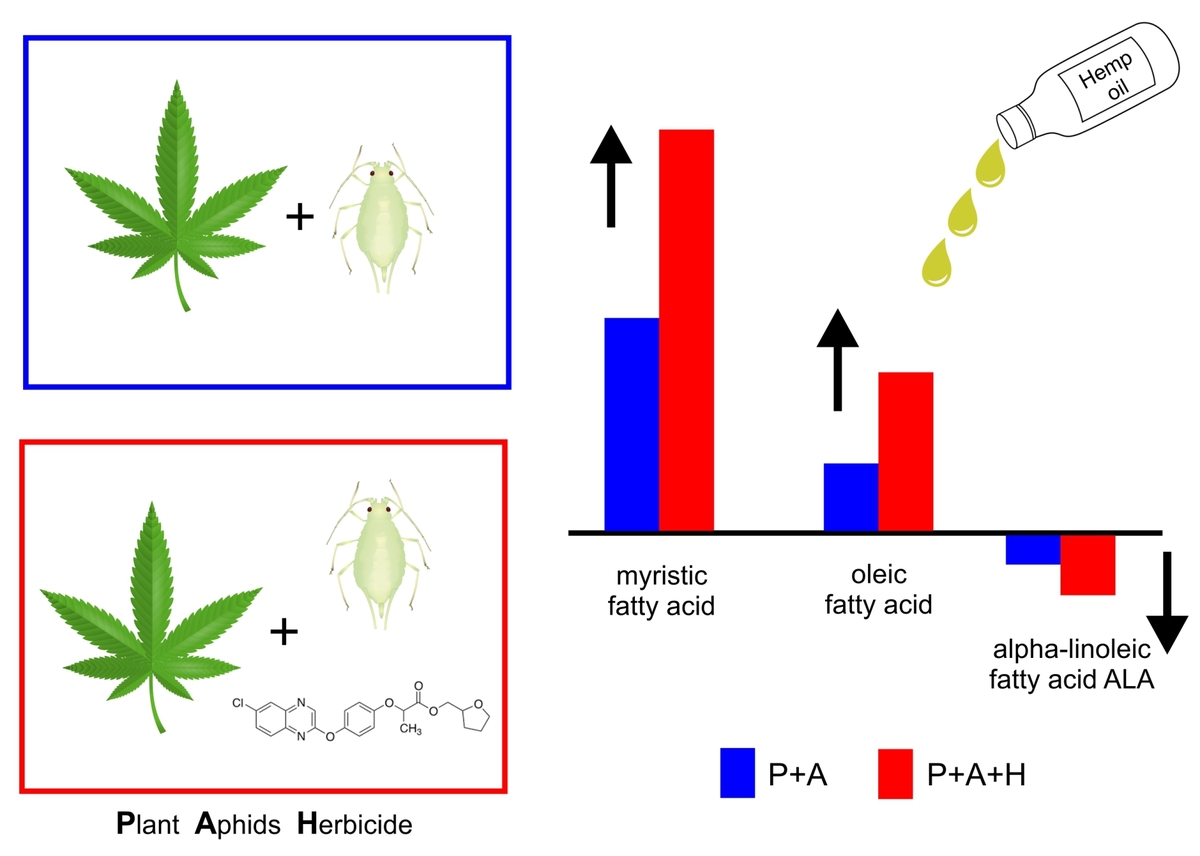
Of 21 FAs detected in hemp leaves, aphid feeding significantly increased the amounts of
myristic and oleic acids and decreased the content of α-linolenic acid. This effect was found
when aphids fed on hemp plants and especially when plants were treated with an herbicide
containing quizalofop-P-tefuryl. This compound on its own did not affect the FA composition.
In spite of the extremely high increase of myristic acid (7- to 9-fold, depending on the
experiment variant), which could cause the repellent effect in hemp plants, the decreased
amount of α-linolenic acid, the precursor of jasmonic acid may have helped aphids to manipulate
the jasmonate signaling pathway involved in plant defense to herbivory enabling
their continued feeding on hemp. This study revealed the importance of FAs in plant defense
as well as the side effects of non-target plant protection products. Future pest management
should take into account the complex interactions between crop plants, their pests
and non-target effects of chemicals used in real field situations.
ACKNOWLEDGEMENTS
The authors would like to thank Paulina Książek-Trela,
Magdalena Wlaszczyk and Katarzyna Beczek for their
valuable technical help.
RESPONSIBLE EDITOR
Milan Brankov
CONFLICT OF INTEREST
The authors have declared that no conflict of interests exist.
REFERENCES (73)
1.
Abay G., Altun M., Karakoç Ö.C., Gül F., Demirtas I. 2013. Insecticidal activity of fatty acid-rich Turkish bryophyte extracts against Sitophilus granarius (Coleoptera: Curculionidae). Combinatorial Chemistry & High Throughput Screening 16 (10): 806–816. DOI: https://doi.org/10.2174/138620....
2.
Aguilar-Marcelino L., Pineda-Alegría J.A., Salinas-Sánchez D.O., Hernández-Velázquez V.M., Silva-Aguayo G.I., Navarro-Tito N., Sotelo-Leyva C. 2022. In vitro insecticidal effect of commercial fatty acids, β-sitosterol, and rutin against the sugarcane aphid, Melanaphis sacchari Zehntner (Hemiptera: Aphididae). Journal of Food Protection 85 (4): 671–675. DOI: https://doi.org/10.4315/JFP-21....
3.
Bakro F., Jędryczka M., Wielgusz K., Sgorbini B., Inchingolo R., Cardenia V. 2020. Simultaneous determination of terpenes and cannabidiol in hemp (Cannabis sativa L.) by fast gas chromatography with flame ionization detection. Journal of Separation Science 43 (14): 2817–2826. DOI: http://doi.org/10.1002/jssc.20....
4.
Bakro F., Wielgusz K., Bunalski M. Jędryczka M. 2018. An overview of pathogen and insect threats to fibre and oilseed chemp (Cannabis sativa L.) and methods for their biocontrol. Integrated Control in Oilseed Crops IOBC-WPRS Bulletin 136: 9–20. https://www.cabidigitallibrary....
5.
Blackman R.L., Eastop V.F. 2024. Aphids of the World’s Plants: An Online Identification and Information Guide. [Available from: http://www.aphidsonworldsplant...] [Accessed 15 May 2024].
6.
Blée E. 2002. Impact of phyto-oxylipins in plant defense. Trends of Plant Science 7: 315–322. DOI: https://doi.org/10.1016/S1360-....
7.
Bergman D.K., Dillwith J.W., Berberet R.C. 1991. Spotted alfalfa aphid, Therioaphis maculata, fatty acids relative to the condition and susceptibility of its host. Archives of Insect Biochemistry and Physiology 18: 1‒12. DOI: https://doi.org/10.1002/arch.9....
8.
Bos J.I.B., Prince D., Pitino M., Maffei M.E., Win J., Hogenhout SA (2010) A Functional Genomics Approach Identifies Candidate Effectors from the Aphid Species Myzus persicae (Green Peach Aphid). PLoS Genetics 6 (11): e1001216. DOI: https://doi.org/10.1371/journa....
9.
Bromilow R., Chamberlain K., Evans A. 1990. Physicochemical aspects of phloem translocation of herbicides. Weed Science 38 (3): 305–314. DOI: https://doi.org/10.1017/S00431....
10.
Callaway J.C. 2004. Hempseed as a nutritional resource - an overview. Euphytica 140 (1–2): 65–72. DOI: http://doi.org/10.1007/s10681-....
11.
Cantele C., Bertolino M., Bakro F., Giordano M., Jędryczka M., Cardenia V. 2020. Antioxidant effects of hemp (Cannabis sativa L.) inflorescence extract in stripped linseed oil. Antioxidants 9 (11): 1131. DOI: https://doi.org/10.3390/antiox....
12.
Chao H., Yu-Ting L., Yu-Xi L., Ge-Fei H., Xue-Qing Y. 2023. Molecular interaction network of plant-herbivorous insects. Advanced Agrochemistry 3 (1): 74‒82. DOI: https://doi.org/10.1016/j.aac.....
13.
Christensen S.A., Kolomiets M.V. 2011. The lipid language of plant-fungal interactions. Fungal Genetics and Biology 48: 4–14. DOI: http://doi.org/10.1016/j.fgb.2....
14.
Cohen E., Levinson Z. 1972. The effect of fatty acids on reproduction of the hide beetle Dermestes maculatus (Dermestidae; Coleoptera). Life Sciences 11: 293‒299. DOI: http://10.1016/0024-3205(72)90....
15.
Cornelissen J.H.C., Lavorel S., Garnier E., Díaz S., Buchmann N., Gurvich D.E., Reich P.B., ter Steege H., Morgan H.D., van der Heijden M.G.A., Pausas J.G., Poorter H. 2003. A handbook of protocols for standardized and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51: 335–380. DOI: http://dx.doi.org/10.1071/BT02....
16.
Cranshaw W., Halbert S., Favret C., Britt K. Miller G. 2018. Phorodon cannabis Passerini (Hemiptera: Aphididae), a newly recognized pest in North America found on industrial cannabis. Insecta Mundi 662: 1–12. DOI: http://doi.org/10.5281/zenodo.....
17.
da Silva M.R.M., Ricci-Júnior E. 2020. An approach to natural insect repellent formulations: from basic research to technological development. Acta Tropica 2020 212: 105419. DOI: http://doi.org/10.1016/j.actat....
18.
de Vos M., Kim J.H., Jander G. 2007. Biochemistry and molecular biology of Arabidopsis-aphid interactions. Bioassays 9 (9): 871–83. DOI: http://doi.org/10.1002/bies.20....
19.
Dobermann D., Field L.M., Michaelson L.V. 2019. Impact of heat processing on the nutritional content of Gryllus bimaculatus (black cricket). Nutrition Bulletin 2: 116–122. DOI: https://doi.org/10.1111/nbu.12....
20.
Document N° SANTE 11312/2021. 2021. Analytical quality control and method validation procedures for pesticide residues analysis in food and feed. European Commission Directorate-General for Health and Food Safety. [Available from: https://www.eurl-pesticides.eu...] [Accessed 25 January 2024].
21.
Durak R., Jedryczka M., Czajka B., Dampc J., Wielgusz K. Borowiak-Sobkowiak B. 2021. Mild Abiotic Stress Affects Development and Stimulates Hormesis of Hemp Aphid Phorodon cannabis. Insects 12 (5): 420. DOI: https://doi.org/10.3390/insect....
22.
Elzinga D.A., De Vos M., Jander G. 2014. Suppression of plant defences by a Myzus persicae (green peach aphid) salivary effector protein. Molecular Plant Microbe Interactions 27 (7): 747–56. DOI: http://doi.org/10.1094/MPMI-01....
23.
European Food Safety Authority. 2008. Conclusion on the Peer Review of Quizalofop-P. European Food Safety Authority Scientific Report 205: 1–216. [Available from: https://efsa.onlinelibrary.wil...] [Accessed 14 January 2024] DOI: https://doi.org/10.2903/j.efsa....
24.
Farag M., Ahmed M.H., Yousef H., Abdel-Rahman A.A. 2011. Repellent and insecticidal activities of Melia azedarach L. against cotton leafworm, Spodoptera littoralis (Boisd.). Zeitschrift für Naturforschung section C Journal of Biosciences 66 (3-4): 129‒35. DOI: http://doi.org/10.1515/znc-201....
25.
Farinon B., Molinari R., Costantini L., Merendino N. 2020. The seed of industrial hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2 (7): 1935. DOI: http://doi.org/10.3390/nu12071....
26.
Farmer E.E., Alméras E., Krishnamurthy V. 2003. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Current Opinion in Plant Biology 6 (4): 372‒378. DOI: http://doi.org/10.1016/s1369-5....
27.
Fogleman J.C., Kircher H.W. 1986. Differential effects of fatty acid chain length on the viability of two species of cactophilic Drosophila. Comparative Biochemistry and Physiology – Part A: Physiology 83 (4): 761–764. DOI: https://doi.org/10.1016/0300-9....
28.
Golimowski W., Teleszko M., Marcinkowski D., Kmiecik D., Grygier A., Kwasnica A. 2022. Quality of oil pressed from hemp seed varieties: ‘Earlina 8FC’, ‘Secuieni Jubileu’ and ‘Finola’. Molecules 27: 3171. DOI: https://doi.org/10.3390/molecu....
29.
Hammer O., Harper D.A.T., Ryan P.D. 2002. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 1–9. DOI: http://palaeo-electronica.org/....
30.
Harwood J.L. 1988. Fatty Acid Metabolism. Annual Review of Plant Biology 39: 101–138. DOI: http://doi.org/10.1146/annurev....
31.
He M., Ding N.Z. 2020. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Frontiers in Plant Science 11: 562785. DOI: https://doi.org/10.3389/fpls.2....
32.
He M., He C.Q., Ding N.Z. 2018. Abiotic stresses: general defenses of land plants and chances for engineering multistress tolerance. Frontiers in Plant Science 9: 1771. DOI: https://doi.org/10.3389/fpls.2....
33.
He M., Qin Chun-Xue, Wang Xu, Ding Nai-Zheng. 2020. Plant Unsaturated Fatty Acids: Biosynthesis and Regulation. Frontiers in Plant Science. 11. DOI: http://doi.org/10.3389/fpls.20....
34.
Heie O.E. 1994. The Aphidoidea (Hemiptera) of Fennoscandia and Denmark. V, Family Aphididae, Part 2 of Tribe Macrosiphini of Subfamily Aphidinae. 1st ed. Brill, Leiden, The Netherlands, 242 pp. DOI: https://archive.org/details/en....
35.
Huizhi L., Yubo Z. 2017. Preparation method of controlled-release natural plant mosquito repellent. CN Patent 102016000854902.
36.
Juárez M.P., Napolitano R. 2000. Effects of organic acids on lipid synthesis and ecdysis in Triatoma infestans eggs. Comparative Biochemistry and Physiology part B: Biochemistry & Molecular Biology 125: 503–510. DOI: https://doi.org/10.1016/S0305-....
37.
Kachroo P., Shanklin J., Shah J., Whittle E.J., Klessig D.F. 2001. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proceedings of the National Academy of Sciences of the United States of America 98: 9448–9453. DOI: http://doi.org/10.1073/pnas.15....
38.
Kanobe C., McCarville M.T., O’Neal M.E., Tylka G.L., MacIntosh G.C. 2015. Soybean Aphid Infestation Induces Changes in Fatty Acid Metabolism in Soybean. PLoS ONE 10 (12): e0145660. DOI: http://doi.org/10.1371/journal....
39.
Kim H.U. 2020. Lipid Metabolism in Plants. Plants 9 (7): 871. DOI: https://doi.org/10.3390/plants....
40.
Kumar S. 2020. Aphid-Plant Interactions: Implications for pest management. plant communities and their environment. IntechOpen. DOI: http://doi.org/10.5772/intecho....
41.
Lemberkovics E., Veszki P., Verzarpetri G. Trka A. 1979. Contributions to the essential oil composition of the flowers and leaves of Cannabis-sativa L. Medicinal Plants of the World (Humana), vol. 3. Ed. Ivan A Ross, Humana Press, USA, 648 pp.
42.
Leonard W., Zhang P., Ying D., Fang Z.X. 2020. Hempseed in food industry: Nutritional value, health benefits, and industrial applications. Comprehensive Reviews in Food Science and Food Safety 19 (1): 282–308. DOI: http://doi.org/10.1111/1541-43....
43.
Li J., Galla A., Avila C.A., Flattmann K., Vaughn K., Goggin F.L. 2021. Fatty acid desaturases in the chloroplast and endoplasmic reticulum promote susceptibility to the green peach aphid Myzus persicae in Arabidopsis thaliana. Molecular Plant Microbe Interactions 34 (6): 691‒702. DOI: http://doi.org/10.1094/MPMI-12....
44.
Liao P, Lechon T, Romsdahl T, Woodfield H, Fenyk S, Fawcett T, Wallington E., Bates R.E., Chye M.L., Chapman K.D. Harwood J.L, Scofield S. 2022. Transgenic manipulation of triacylglycerol biosynthetic enzymes in B. napus alters lipid-associated gene expression and lipid metabolism. Scientific Reports 12: 3352. DOI: https://doi.org/10.1038/s41598....
45.
Lim G.H., Singhal R., Kachroo A., Kachroo P. 2017. Fatty acid- and lipid-mediated signaling in plant defense. Annual Review of Phytopathology 55: 505–536. DOI: http://doi.org/10.1146/annurev....
46.
Louis J., Leung Q., Pegadaraju V., Reese J., Shah J. 2010. PAD4-dependent antibiosis contributes to the ssi2-conferred hyper-resistance to the green peach aphid. Molecular Plant Microbe Interactions 23 (5): 618–627. DOI: http://doi.org/10.1094/MPMI-23....
47.
Louis J., Shah J. 2013. Arabidopsis thaliana – Myzus persicae interaction: shaping the understanding of plant defense against phloem-feeding aphids. Frontiers in Plant Science 4: 213. DOI: https://doi.org/10.3389/fpls.2....
48.
Lupette J., Benning C. 2020. Human health benefits of very-long-chain polyunsaturated fatty acids from microalgae. Biochimie 178: 15–25. DOI: https://doi.org/10.1016/j.bioc....
49.
MacWilliams J., Peirce E., Pitt W.J., Schreiner M., Matthews T., Yao L., Broeckling C., Nachappa P. 2023. Assessing the adaptive role of cannabidiol (CBD) in Cannabis sativa defense against cannabis aphids. Frontiers in Plant Science 14: 1223894. DOI: https://doi.org/10.3389/fpls.2....
50.
Mathew N., Ayyanar E., Shanmugavelu S., Muthuswamy K. 2013. Mosquito attractant blends to trap host seeking Aedes aegypti. Parasitology Research 112 (3): 1305‒12. DOI: https://doi.org/10.1007/s00436....
51.
McCauley J.I., Meyer B.J., Winberg P.C., Skropeta D. 2016. Parameters affecting the analytical profile of fatty acids in the macroalgal genus Ulva. Food Chemistry 209: 332–340. DOI: http://doi.org/10.1016/j.foodc....
52.
McFarlane J.E., Henneberry G. O. 1965. Inhibition of the growth of an insect by fatty acids. Journal of Insect Physiology 11 (9): 1247‒1252. DOI: https://doi.org/10.1016/0022-1....
53.
McPartland J.M., Clarke R.C., Watson D.P. 2000. Hemp Diseases and Pests Management and Biocontrol. 1st ed. CABI International, CABI Publishing: Wallingford, Oxon, UK, 251 pp.
54.
Morkunas I., Mai V.C., Gabryś B. 2011. Phytohormonal signalling in plant responses to aphid feeding. Acta Physiologiae Plantarum 33: 2057–2073. DOI: http://doi.org/10.1007/s11738-....
55.
Mukhopadhyay S., Bhattacharyya A., Das S. 2012. Fate and persistence of herbicide quizalofop-p-tefuryl on black gram. Journal of Crop and Weed 8 (1): 190‒192. DOI: https://www.cropandweed.com/vo....
56.
Nair K.S.S., Desai A.K. 1972. Some new findings on factors inducing diapause in Trogoderma granarium Everts (Coleoptera, Dermestidae). Journal of Stored Products Research 8: 27‒54. DOI: https://doi.org/10.1016/0022-4....
57.
Nalam V., Louis J., Shah J. 2018. Plant defense against aphids, the pest extraordinaire. Plant Science 279: 96–107. DOI: https://doi.org/10.1016/j.plan....
58.
Nalam V.J., Keeretaweep J., Sarowar S., Shah J. 2012. Root-derived oxylipins promote green peach aphid performance on Arabidopsis foliage. The Plant Cell 22 (4): 1643–1653. DOI: https://doi.org/10.1105/tpc.11....
59.
Ohlrogge J.B. 1994. Design of new plant products: engineering of fatty acid metabolism. Plant Physiology 104: 821‒826. DOI: http://doi.org/10.1104/pp.104.....
60.
Pitino M., Hogenhout S.A. 2013. Aphid protein effectors promote aphid colonization in a plant species-specific manner. Molecular Plant Microbe Interactions 26 (1): 130–139. DOI: http://doi.org/10.1094/MPMI-07....
61.
Podbielska M., Kus-Liśkiewicz M., Jagusztyn B., Szpyrka E. 2023. Effect of microorganisms on degradation of fluopyram and tebuconazole in laboratory and field studies. Environmental Science and Pollution Research 30 (16): 1‒15. DOI: https://doi.org/10.1007/s11356....
62.
Prost I., Dhondt S., Rothe G., Vicente J., Rodriguez M.J., Kift N. et al. 2005. Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiology 139: 1902–1913. DOI: http://doi.org/10.1104/pp.105.....
63.
Sivakumar R., Jebanesan A., Govindarajan M., Rajasekar P. 2011. Larvicidal and repellent activity of tetradecanoic acid against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.) (Diptera: Culicidae). Asian Pacific Journal of Tropical Medicine 4 (9): 706‒710. DOI: http://doi.org./10.1016/S1995-....
64.
Słowik-Borowiec M., Szpyrka E., Książek-Trela P., Podbielska M. 2022. Simultaneous Determination of Multi-Class Pesticide Residues and PAHs in Plant Material and Soil Samples Using the Optimized QuEChERS Method and Tandem Mass Spectrometry Analysis. Molecules 27: 2140. DOI: https://doi.org/10.3390/molecu....
65.
Smith B.C. 2011. Fundamentals of Fourier Transform Infrared Spectroscopy. 2nd ed. CRC Press; Taylor & Francis Group, Boca Raton, London, New York, 207 pp. DOI: https://doi.org/10.1201/b10777.
66.
Sotelo-Leyva C., Salinas-Sánchez D.O., Peña-Chora G., Trejo-Loyo A.G., González-Cortázar M., Zamilpa A. 2020. Insecticidal compounds in Ricinus communis L. (Euphorbiaceae) to control Melanaphis sacchari Zehntner (Hemiptera: Aphididae). Florida Entomologist 103: 91–95. DOI: https://doi.org/10.1653/024.10....
67.
Storelli M. 2014. Evaluation of toxic metal (Hg, Cd, Pb), polychlorinated biphenyl (PCBs), and pesticide (DDTs) levels in aromatic herbs collected in selected areas of Southern Italy. Environmental Science and Pollution Research 21: 946–953. DOI: http://doi.org/10.1007/s11356-....
68.
Szpyrka E., Broda D., Oklejewicz B., Podbielska M., Słowik-Borowiec M., Jagusztyn B., Chrzanowski G., Kus-Liskiewicz M., Duda M., Zuczek J., Wnuk M., Lewińska A. 2020. A Non-Vector Approach to Increase Lipid Levels in the Microalga Planktochlorella nurekis. Molecules 25 (2): 270. DOI: https://doi.org/10.3390/molecu....
69.
Truta E., Surdu S., Rosu C.M., Asaftei M. 2009. Hemp -Biochemical diversity and multiple uses. Analele Stiintifice ale Universitatii Al. I Cuza din Iasi. Genetica si Biologie Moleculara 10: 1‒8. [Available on: https://www.researchgate.net/p...].
70.
Wu J., Baldwin IT. 2010. New insights into plant responses to the attack from insect herbivores. Annual Review of Genetics. 44:1‒24. DOI: https://doi.org/10.1146/annure....
71.
Wychen S., Ramirez K., Laurens L.M.L. 2013. Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by in situ Transesterification. Laboratory Analytical Procedure (LAP) DOI: https://doi.org/10.2172/111808....
72.
Zhao Y., Cao P., Cui Y., Liu D., Li J., Zhao Y., Yang S., Zhang B., Zhou R., Sun M., Guo X., Yang M., Xin D., Zhang Z., Li X., Lv Ch., Liu Ch., Qi Z., Xu J., Wu X., Chen Q. 2021. Enhanced production of seed oil with improved fatty acid composition by overexpressing NAD+-dependent glycerol-3-phosphate dehydrogenase in soybean. Journal of Integrative Plant Biology 63: 1036–1053. DOI: https://doi.org/10.1111/jipb.1....
73.
Zhou X., Ling X., Guo H., Zhu-Salzman K., Ge F., Sun Y. 2021. Serratia symbiotica Enhances Fatty Acid Metabolism of Pea Aphid to Promote Host Development. International Journal of Molecular Sciences 22 (11): 5951. DOI: https://doi.org/10.3390/ijms22....
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.