RAPID COMMUNICATION
Effect of potato tuber greening on blackleg development by Dickeya solani and Pectobacterium brasiliense
1
Młochów Division, Plant Breeding and Acclimatization Institute – National Research Institute in Radzików, Młochów, Poland
A - Research concept and design; B - Collection and/or assembly of data; C - Data analysis and interpretation; D - Writing the article; E - Critical revision of the article; F - Final approval of article
Submission date: 2024-09-17
Acceptance date: 2024-10-22
Online publication date: 2025-06-10
Corresponding author
Anna Maria Grupa-Urbańska
Młochów Division, Plant Breeding and Acclimatization Institute – National Research Institute in Radzików, Młochów, Poland
Młochów Division, Plant Breeding and Acclimatization Institute – National Research Institute in Radzików, Młochów, Poland
Journal of Plant Protection Research 2025;65(2):274-279
HIGHLIGHTS
- Seed tuber greening increases glycoalkaloid content
- Seed tuber greening can be non-chemical strategy for blackleg control
- Seed tuber greening significantly reduces Dickeya solani infection in potato plants
KEYWORDS
TOPICS
ABSTRACT
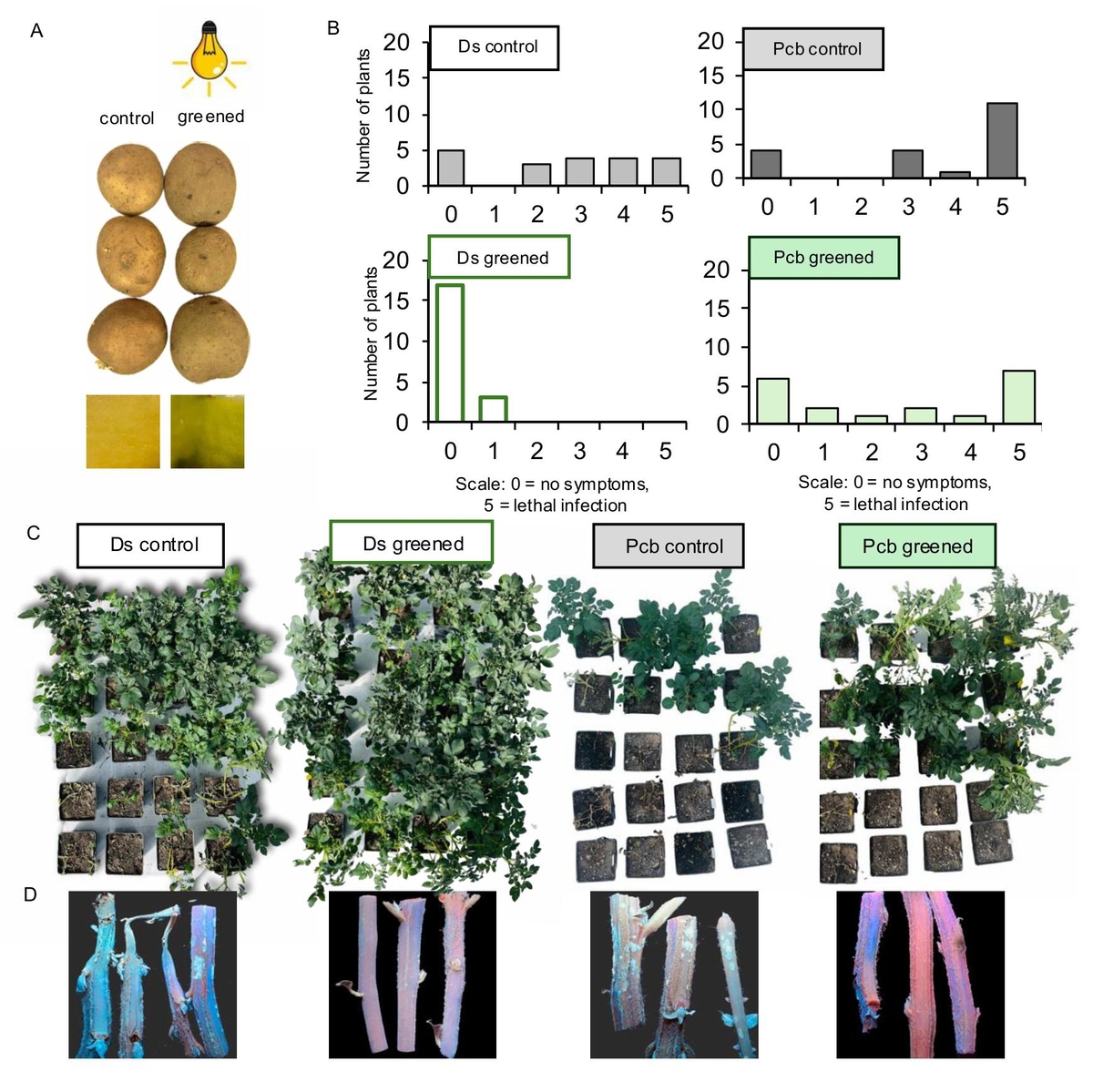
Potato (Solanum tuberosum) is a globally important crop, but its production is often threatened
by pectinolytic bacteria of genus Pectobacterium and Dickeya, including Pectobacterium
brasiliense (Pcb), and Dickeya solani (Ds), which cause two diseases, soft rot of potato
tubers and blackleg of potato plants. These pathogens cause a reduction of potato yield, and
significant yield losses due to tuber rot in storage. Currently, there are no effective chemical
solutions to control these bacterial pathogens. This study aimed to investigate the effect of
tuber greening, a process that significantly increases the content of glycoalkaloids (GAs),
on the susceptibility of the potato cultivar Tajfun to infection by Pcb and Ds. Tubers were
exposed to continuous artificial light for 2 weeks to induce greening. Control tubers were
kept in the dark under the same environmental conditions. Then, tubers were infiltrated
with Pcb and Ds under low pressure to ensure efficient bacterial penetration and planted
in pots under controlled conditions. After 3 weeks phenotypic symptoms of bacterial infection
such as wilting, overall plant vitality and stem necrosis were determined. Results
showed a significant reduction in Ds infection in greened tubers compared to non-greened
controls, supporting the hypothesis that greening which increases GAs levels, enhances
resistance to bacterial pathogens. The response to Pcb was more variable, with some plants
grown from greened tubers still exhibiting high levels of infection, suggesting that while
greening may reduce susceptibility, the greater aggressiveness of Pcb may limit the protective
effects of greening. In conclusion, the present study showed that tuber greening
could be an effective non-chemical method for controlling blackleg, particularly against
Ds. However, the variable response to Pcb indicates that additional strategies are needed.
Future research should focus on integrating GAs-based defenses with potato cultivars that
exhibit stronger resistance to pectinolytic bacteria for improved management of blackleg.
ACKNOWLEDGEMENTS
We are grateful
to Professor E. Lojkowska for kindly providing the
strain Dickeya solani IFB0099 from the collection of
the Intercollegiate Faculty of Biotechnology University
of Gdańsk and the Medical University of Gdańsk, Poland.
We wish to thank Professor Dariusz Mańkowski
for his statistical support.
FUNDING
This research was financially supported by a grant
from the Ministry of Agriculture and Rural Development,
Poland, Basic Research for Biological Progress
in Plant Production, Task number 28.
RESPONSIBLE EDITOR
Giovani Zabot
CONFLICT OF INTEREST
The authors have declared that no conflict of interests exist.
REFERENCES (32)
1.
Al Kabee H.J.J. 2019. Antimicrobial activity of glycoalkaloids extracted from potato peels. Life Science Archives 5: 1624–1629. DOI: 10.22192/lsa.2018.5.3.2.
2.
Andrivon D., Corbière R., Lucas J.-M., Pasco C., Gravoueille J.-M., Pellé R., Dantec J.-P., Ellissèche D. 2003. Resistance to late blight and soft rot in six potato progenies and glycoalkaloid contents in the tubers. American Journal of Potato Research 80 (2): 125–134. DOI: https://doi.org/10.1007/BF0287....
3.
Charkowski A., Sharma K., Parker M.L., Secor G.A., Elphinstone J. 2020. Bacterial diseases of potato. p. 351–388. In: “The Potato Crop” (Campos H., Ortiz O., eds.). Springer International Publishing. DOI: https://doi.org/10.1007/978-3-....
4.
Czajkowski R., Pérombelon M.C.M., Van Veen J.A., Van Der Wolf J.M. 2011. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathology 60 (6): 999–1013. DOI: https://doi.org/10.1111/j.1365....
5.
Dahlin P., Müller M.C., Ekengren S., McKee L.S., Bulone V. 2017. The impact of steroidal glycoalkaloids on the physiology of Phytophthora infestans, the causative agent of potato late blight. Molecular Plant-Microbe Interactions 30 (7): 531542. DOI: https://doi.org/10.1094/MPMI-0....
6.
Dubois Gill E., Schaerer S. and Dupuis B. 2014. Factors impacting blackleg development caused by Dickeya spp. in the field. European Journal of Plant Pathology 140: 317–327.
7.
Dupuis B., Nkuriyingoma P., Van Gijsegem F. 2021. Economic impact of Pectobacterium and Dickeya species on potato crops: a review and case study. 263–282. In: “Plant Diseases Caused by Dickeya and Pectobacterium Species” (Van Gijsegem, F., Van Der Wolf J.M., Toth I.K., eds.). Springer International Publishing, Cham. DOI: https://doi.org/10.1007/978-3-....
8.
Fewell A.M., Roddick J.G. 1993. Interactive antifungal activity of the glycoalkaloids α-solanine and α-chaconine. Phytochemistry 33 (2): 323–328. DOI: https://doi.org/10.1016/0031-9....
9.
Friedman M. 2006. Potato glycoalkaloids and metabolites: roles in the plant and in the diet. Journal of Agricultural and Food Chemistry 54 (23): 8655–8681. DOI: https://doi.org/10.1021/jf0614....
10.
Friedman M., McDonald G.M., Filadelfi-Keszi M. 1997. Potato glycoalkaloids: chemistry, analysis, safety, and plant physiology. Critical Reviews in Plant Sciences 16 (1): 55–132. DOI: https://doi.org/10.1080/073526....
11.
Ginzberg I., Barel G., Ophir R., Tzin E., Tanami Z., Muddarangappa T., de Jong W., Fogelman E. 2009. Transcriptomic profiling of heat-stress response in potato periderm. Journal of Experimental Botany 60 (15): 44114421. DOI: 10.1093/jxb/erp281.
12.
Golanowska M., Galardini M., Bazzicalupo M., Hugouvieux-Cotte-Pattat N., Mengoni A., Potrykus M., Slawiak M., Lojkowska E. 2015. Draft genome sequence of a highly virulent strain of the plant pathogen Dickeya solani, IFB0099. Genome Announcements 3 (2): e0010915. DOI: https://doi.org/10.1128/genome....
13.
Grunenfelder L.A., Knowles L.O., Hiller L.K., Knowles N.R. 2006. Glycoalkaloid development during greening of fresh market potatoes (Solanum tuberosum L.). Journal of Agricultural and Food Chemistry 54 (16): 5847–5854. DOI: https://doi.org/10.1021/jf0607....
14.
Hélias A.J. 2000. Development of symptoms caused by Erwinia carotovora ssp. atroseptica under field conditions and their effects on the yield of individual potato plants. Plant Pathology 49 (1): 23–32. DOI: https://doi.org/10.1046/j.1365....
15.
Hélias V., Hamon P., Huchet E., Wolf J.V.D., Andrivon D. 2012. Two new effective semiselective crystal violet pectate media for isolation of Pectobacterium and Dickeya. Plant Pathology 61: 339345.DOI: https://doi.org/10.1111/j.1365....
16.
Keukens E.A., de Vrije T., van den Boom C., de Waard P., Plasman H.H., Thiel F., Chupin V., Jongen W.M., de Kruijff B. 1995. Molecular basis of glycoalkaloid induced membrane disruption. Biochimica et Biophysica Acta 1240: 216–228. DOI: https://doi.org/10.1016/0005-2....
17.
Lebecka R., Kistowski M., Dębski J., Szajko K., Murawska Z., Marczewski W. 2019. Quantitative proteomic analysis of differentially expressed proteins in tubers of potato plants differing in resistance to Dickeya solani. Plant and Soil. 441: 317–329. DOI: https://doi.org/10.1007/s11104....
18.
Lebecka R., Michalak K. 2020. Laboratory assessment of aggressiveness of pectinolytic bacteria isolated from stems and potato tubers showing disease symptoms. Ziemniak Polski 4: 33–39. (in Polish).
19.
Lelario F., Scrano L., De Franchi S., Bonomo M.G., Salzano G., Milan S., Milella L., Bufo S.A. 2018. Identification and antimicrobial activity of most representative secondary metabolites from different plant species. Chemical and Biological Technologies in Agriculture 5 (1): 13. DOI: https://doi.org/10.1186/s40538....
20.
Motyka-Pomagruk A., Babinska-Wensierska W., Sledz W., Kaczorowska A.K., Lojkowska E. 2023. Phyloproteomic study by MALDI-TOF MS in view of intraspecies variation in a significant homogenous phytopathogen Dickeya solani. Scientific Reports 13 (1): 18863. DOI: https://doi.org/10.1038/s41598....
21.
Muraja-Fras J., Krsnik-Rasol M., Wrischer M. 1994. Plastid transformation in greening potato tuber tissue. Journal of Plant Physiology 144 (1): 58–63. DOI: https://doi.org/10.1016/S0176-....
22.
Nahirñak V., Almasia N.I., González M.N., Massa G.A., Décima Oneto C.A., Feingold S.E., Hopp H.E., Vazquez Rovere C. 2022. State of the art of genetic engineering in potato: from the first report to its future potential. Frontiers in Plant Science. 12: 768233. DOI: https://doi.org/10.3389/fpls.2....
23.
Okamoto H., Ducreux L.J.M., Allwood J.W., Hedley P.E., Wright A., Gururajan V., Terry M.J., Taylor M.A. 2020. Light regulation of chlorophyll and glycoalkaloid biosynthesis during tuber greening of potato S. tuberosum. Frontiers in Plant Science 11: 753. DOI: https://doi.org/10.3389/fpls.2....
24.
Percival G., Dixon G.R. 1996. Glycoalkaloid concentrations in aerial tubers of potato (Solanum tuberosum L). Journal of the Science of Food and Agriculture 70: 439–448.
25.
Rangarajan A., Miller A. R., Veilleux R. E. 2000. Leptine glycoalkaloids reduce feeding by Colorado potato beetle in diploid Solanum sp. hybrids. The Journal of the American Society for Horticultural Science 125: 689693.
26.
Rymuza K., Gugała M., Zarzecka K., Sikorska A., Findura P., Malaga-Toboła U., Kapela K., Radzka E. 2020. The effect of light exposures on the content of harmful substances in edible potato tuber. Agriculture 10: 139. DOI: https://doi.org/10.3390/agricu....
27.
Sołtys-Kalina D., Grupa-Urbańska A., Lebecka R., Tallant M., Kellenberger I., Dupuis B. 2023. Increase of glycoalkaloid content in potato tubers by greening as a method to reduce the spread of Pectobacterium and Dickeya spp. in seed production systems. Microorganisms 11 (3): 605. DOI: https://doi.org/10.3390/microo....
28.
Toth I. K., Barny M.-A., Brurberg M. B., Condemine G., Czajkowski R. L., Elphinstone J. G., Helias V., Johnson S. B., Moleleki L. N., Pirhonen M., Rossmann S., Tsror L., van der Waals J. E., van der Wolf J. M., van Gijsegem F., Yedidia I. 2021. Pectobacterium and Dickeya: environment to disease development. p . 3984. In: “Plant Diseases Caused by Dickeya and Pectobacterium Species” (van Gijsegem F., van der Wolf J.M., Toth I.K., eds.). Springer. DOI: https://doi.org/10.1007/978-3-....
29.
van der Wolf J.M., Acuña I., de Boer S.H., Brurberg M.B., Cahill G., Charkowski A.O., Coutinho T., Davey T., Dees M.W., Degefu Y., Dupuis B., Elphinstone J.G., Fan J., Fazelisangari E., Fleming T. 2021. Diseases caused by Pectobacterium and Dickeya species around the world. p. 215–261. In: “Plant Diseases Caused by Dickeya and Pectobacterium Species” (van Gijsegem F., van der Wolf J.M., Toth I.K., eds.). Springer. DOI: https://doi.org/10.1007/978-3-....
30.
van Gijsegem F., Toth I.K., Van Der Wolf J.M. 2021. Soft rot Pectobacteriaceae: a brief overview. p. 1–11. In: “Plant Diseases Caused by Dickeya and Pectobacterium Species” (Van Gijsegem, F., Van Der Wolf, J.M., Toth, I.K., eds.). Springer International Publishing, Cham. DOI: https://doi.org/10.1007/978-3-....
31.
Wolters P.J, Wouters D., Tikunov Y.M., Ayilalath S., Kodde L.P., Strijker M.F., Caarls L., Visser R.G.F., Vleeshouwers V.G.A. 2023. Tetraose steroidal glycoalkaloids from potato provide resistance against Alternaria solani and Colorado potato beetle. eLife 12, Article RP87135. DOI: https://doi.org/10.7554/eLife.....
32.
Zhang C., Chen W., Wang B., Wang Y., Li N., Li R., Yan Y., Sun Y., He J. 2024. Potato glycoside alkaloids exhibit antifungal activity by regulating the tricarboxylic acid cycle pathway of Fusarium solani. Frontiers in Microbiology 15 (15): 1390269. DOI: 10.3389/fmicb.2024.1390269.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.