REVIEW
Plant viral disease management-from cross protection to CRISPR
1
Department of Plant Protection, Faculty of Agriculture, University of Tabriz, Tabriz, Iran
2
Department of Plant Pathology, Faculty of Agriculture, University of Tarbiat Modares, Tehran, Iran
A - Research concept and design; B - Collection and/or assembly of data; C - Data analysis and interpretation; D - Writing the article; E - Critical revision of the article; F - Final approval of article
Submission date: 2024-05-09
Acceptance date: 2024-08-28
Online publication date: 2025-06-16
Corresponding author
Nemat Sokhandan-Bashir
Department of Plant Protection, Faculty of Agriculture, University of Tabriz, Tabriz, Iran
Department of Plant Protection, Faculty of Agriculture, University of Tabriz, Tabriz, Iran
Journal of Plant Protection Research 2025;65(2):145-163
HIGHLIGHTS
- Biotechnological-based viral plant disease management methods are the most efficient
- Strategies for resistance engineering to plant viruses are constantly evolving
- Syn-tasiRNA and CRISPR are the most efficient methods for creating resistant plants
KEYWORDS
TOPICS
ABSTRACT
In contrast to other plant pathogens, the control of viruses through chemical compounds
is not feasible. Consequently, the management of plant viruses has predominantly relied
on biotechnological approaches rather than those used for other pathogens. This paper
presents a thorough review that takes into account an extensive literature analysis to offer
a comprehensive understanding of biotechnological strategies aimed at developing stable
engineered virus-resistant plants. Examples of these strategies were highlighted in crops,
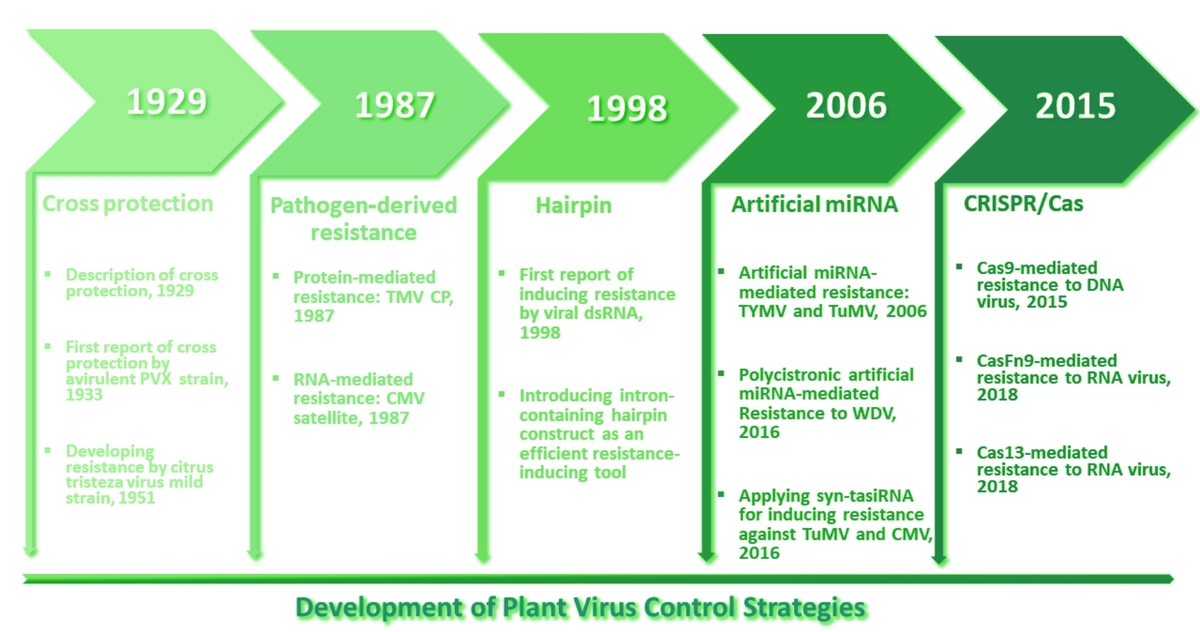
using as many cases as were available, where these strategies had been used, including cross-
-protection, pathogen-derived resistance (PDR), hairpin RNA, artificial small RNAs, and
genome editing-based CRISPR/Cas systems. In evaluating the trends over time, we have
critically assessed the advantages and disadvantages of each approach, identifying synthetic
trans-acting microRNA (syn-tasiRNA) and CRISPR/Cas as the most effective and precise
methods exhibiting minimal off-target effects on the plant genome. Furthermore, we have
discussed the emerging innovations in non-transgenic resistance strategies such as the application
of double-stranded (ds) RNA which hold promise for overcoming the significant
challenges associated with the commercialization of engineered resistant plants.
ACKNOWLEDGEMENTS
The authors would like to thank Hussein Golafrouz
for his invaluable help in the preparation of Figures 2
and 3.
RESPONSIBLE EDITOR
Julia Minicka
CONFLICT OF INTEREST
The authors have declared that no conflict of interests exist.
REFERENCES (154)
1.
Abdalla O. A Eraky A.I., Mohamed S.A., Fahmy F.G., 2018. Management of potato virus Y (PVY-NTN) causing PTNRD disease in potato by prior treatment with a mild PVY strain, Journal of Plant Protection Research 58 (2): 130–136. DOI: https://doi.org/10.24425/11913....
2.
Agrios G.N. 2005. Plant Pathology .5th Edition. Academic Press, London, New York, 922 pp.
3.
Ahmad A., Jamil A., Munawar N. 2023. GMOs or Non-GMOs? The CRISPR Conundrum. Frontier Plant Science 14: 1232938. DOI: https://doi.org/10.3389/fpls.2....
4.
Akbarimotlagh M., Azizi A., Shams-Bakhsh M., Jafari M., Ghasemzadeh A., Palukaitis P. 2023. Critical points for the design and application of RNA silencing constructs for plant virus resistance. Advances in Virus Research 115: 159–203. DOI: https://doi.org/10.1016/bs.aiv....
5.
Aman R., Ali Z., Butt H., Mahas A., Aljedaani F., Khan M.Z., Ding S., Mahfouz M. 2018a. RNA virus interference via CRISPR/Cas13a system in plants. Genome Biology 19 (1): 1–9. DOI: 10.1186/s13059-017-1381-1
6.
Aman R., Mahas A., Butt H., Ali Z., Aljedaani F., Mahfouz M. 2018b. Engineering RNA virus interference via the CRISPR/Cas13 machinery in Arabidopsis. Viruses 10 (12): 732. DOI: https://doi.org/10.3390/v10120....
7.
Ariga H., Toki S., Ishibashi K. 2020. Potato virus X vector-mediated DNA-free genome editing in plants. Plant Cell Physiology 61 (11): 1946–1953. DOI: https://doi.org/10.1093/pcp/pc....
8.
Bahari A., Castillo A.G., Safaie N., Bejarano E.R., Luna A.P., Shams-Bakhsh M. 2023 BCTIV V3 is a multifunctional protein; silencing suppressor and potential movement protein. 5th International and 17th Iranian Genetic Congress. 6–8th March Shahid Beheshti Universirty, Tehran, Iran.
9.
Bahari A., Castillo A.G., Safaie N., Bejarano E.R., Luna A.P., Shams-Bakhsh M. 2022. Functional analysis of V2 protein of beet curly top Iran virus. Plants 11 (23): 3351. DOI: https://doi.org/10.3390/plants....
10.
Baig M.S., Akhtar S., Khan J.A. 2021. Engineering tolerance to CLCuD in transgenic Gossypium hirsutum cv. HS6 expressing Cotton leaf curl Multan virus-C4 intron hairpin. Scientific Report 11 (1): 14172. DOI:
11.
Bhaya D., Davison M., Barrangou R. 2011. CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annual Review of Genetics 45: 273–297. DOI: https://doi.org/10.1146/annure....
12.
Bleys A., Vermeersch L., van Houdt H., Depicker A. 2006. The frequency and efficiency of endogene suppression by transitive silencing signals is influenced by the length of sequence homology. Plant Physiology 142 (2): 788–796. DOI:
13.
Bonfim K., Faria J.C., Nogueira E.O., Mendes É.A., Aragão F.J. 2007. RNAi-mediated resistance to Bean golden mosaic virus in genetically engineered common bean (Phaseolus vulgaris). Molelcular Plant Microbe Interaction 20 (6): 717726. DOI: https://doi.org/10.1094/MPMI-2....
14.
Bos L. 1999. Plant viruses, unique and intriguing pathogens: a textbook of plant virology. Backhuys Publishers.
15.
Cao Y., Zhou H., Zhou X., Li F. 2020. Control of plant viruses by CRISPR/Cas system-mediated adaptive immunity. Fronter in Microbiology 11: 593700. DOI: https://doi.org/10.3389/fmicb.....
16.
Carbonell A., Daròs J.A. 2017. Artificial microRNAs and synthetic trans‐acting small interfering RNAs interfere with viroid infection. Molecular Plant Phatology 18 (5): 746–753. DOI: https://doi.org/ 10.1111/mpp.12529.
17.
Carbonell A., Lison P., Daròs J.A. 2019a. Multi‐targeting of viral RNAs with synthetic trans‐acting small interfering RNAs enhances plant antiviral resistance. Plant Journal 100 (4): 720–737. DOI: https://doi.org/10.1111/tpj.14....
18.
Carbonell A., López C., Daròs J.-A. 2019b. Fast-forward identification of highly effective artificial small RNAs against different tomato spotted wilt virus isolates. Molecular Plant Microbe Interaction 32 (2): 142–156. DOI: 10.1094/MPMI-05-18-0117-T
19.
Carbonell A., Takeda A., Fahlgren N., Johnson S.C., Cuperus J.T., Carrington J.C. 2014. New generation of artificial MicroRNA and synthetic trans-acting small interfering RNA vectors for efficient gene silencing in Arabidopsis. Plant Physiology 165 (1): 15–29. DOI: https://doi.org/10.1104/pp.113....
20.
Chandrasekaran J., Brumin M., Wolf D., Leibman D., Klap C., Pearlsman M., Sherman A., Arazi T., Gal‐On A. 2016. Development of broad virus resistance in non‐transgenic cucumber using CRISPR/Cas9 technology. Molecular Plant Phatology 17 (7): 1140–1153. DOI: https://doi.org/10.1111/mpp.12....
21.
Chen L., Cheng X., Cai J., Zhan L., Wu X., Liu Q., Wu X. 2016. Multiple virus resistance using artificial trans-acting siRNAs. Jouranl of Virological Methods 228: 16–20. DOI: https://doi.org/ 10.1016/j.jviromet.2015.11.004.
22.
Chen X. 2009. Small RNAs and their roles in plant development. Annual Review of Cell and Developmental 25: 21–44. DOI: https://doi.org/10.1146/annure....
23.
Cisneros A.E. and Carbonell A. 2020. Artificial small RNA-based silencing tools for antiviral resistance in plants. Plants 9 (6): 669. DOI: https://doi.org/10.3390/plants....
24.
Das K., Ayim B.Y., Borodynko-Filas N., Das S.C., Aminuzzaman F.M. 2023, Genome editing (CRISPR/Cas9) in plant disease management: challenges and future prospects. Journal of Plant Protection Research 63 (2) :159–172. DOI: https://doi.org/10.24425/jppr.....
25.
Davis M.J., Ying Z. 2004. Development of papaya breeding lines with transgenic resistance to Papaya ringspot virus. Plant Disease 88 (4): 352358. DOI: https://doi.org/10.1094/PDIS.2....
26.
Culver J.N. 1996. Tobamovirus cross protection using a potexvirus vector. Virology 226 (2): 228–235. DOI: https://doi.org/10.1006/viro.1....
27.
Dalakouras A., Tzanopoulou M., Tsagris M., Wassenegger M., Kalantidis K. 2011. Hairpin transcription does not necessarily lead to efficient triggering of the RNAi pathway. Transgenic Research 20: 293–304. DOI: https://doi.org/10.1007/s11248....
28.
Das P.R. and Sherif S.M. 2020. Application of exogenous dsRNAs-induced RNAi in agriculture: Challenges and triumphs. Frontier Plant Science 11: 946. DOI: https://doi.org/10.3389/fpls.2....
29.
De Zoeten G., Fulton R. 1975. Understanding generates possibilities [Cross protection as a tool in plant virus control]. Phytopaltology 65.
30.
Deb S., Choudhury A., Kharbyngar B., Satyawada R.R. 2022. Applications of CRISPR/Cas9 technology for modification of the plant genome. Genetica 150 (1): 1–12. DOI: https://doi.org/10.1007/s10709....
31.
Duan C., Wang C., Guo H. 2008. Delayed resistance to Cucumber mosaic virus mediated by 3’UTR-derived hairpin RNA. Chinese Science Bulletin 53 (21): 3301–3310. DOI: https://doi.org/10.1007/s11434....
32.
Duan C.-G., Wang C.-H., Guo H.-S. 2012. Application of RNA silencing to plant disease resistance. Silence 3 (1): 1–8. DOI: https://doi.org/10.1186/1758-9....
33.
Fahim M., Millar A.A., Wood C.C., Larkin P.J. 2012. Resistance to Wheat streak mosaic virus generated by expression of an artificial polycistronic microRNA in wheat. Plant Biotecnology Journal 10 (2): 150–163. DOI: https://doi.org/ 10.3389/fpls.2023.1232938.
34.
Fahlgren N., Hill S.T., Carrington J.C., Carbonell A. 2016. P-SAMS: a web site for plant artificial microRNA and synthetic trans-acting small interfering RNA design. Bioinformatics 32 (1): 157–158. DOI: https://doi.org/10.1093/bioinf....
35.
Fire A., Xu S., Montgomery M., Kostas S., Driver S., Mello C. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391 (6669): 806–811.
36.
Filichkin S.A., DiFazio S.P., Brunner A.M., Davis J.M., Yang Z.K., Kalluri U.C., Arias R.S., Etherington E., Tuskan G.A., Strauss S.H. 2007. Efficiency of gene silencing in Arabidopsis: direct inverted repeats vs. transitive RNAi vectors. Plant Biotechnology Journal 5 (5): 615–626. DOI: https://doi.org/10.1111/j.1467....
37.
Flachowsky H., Hanke M.V., Peil A., Strauss S., Fladung M. 2009. A review on transgenic approaches to accelerate breeding of woody plants. Plant Breeding 128 (3): 217–226. DOI: https://doi.org/10.1111/j.1439....
38.
Fondong V.N. 2013. Geminivirus protein structure and function. Moloelcular Plant Phatology 14 (6): 635–649. DOI: https://doi.org/10.1111/mpp.12....
39.
Fuentes A., Carlos N., Ruiz Y., Callard D., Sánchez Y., Ochagavía M.E., Seguin J., Malpica-López N., Hohn T., Lecca M.R. 2016. Field trial and molecular characterization of RNAi-transgenic tomato plants that exhibit resistance to tomato yellow leaf curl geminivirus. Molecular Plant-Microbe Interaction 29 (3): 197–209. DOI: https://doi.org/10.1094/MPMI-0....
40.
Fusaro A.F., Matthew L., Smith N.A., Curtin S.J., Dedic‐Hagan J., Ellacott G.A., Watson J.M., Wang M.B., Brosnan C., Carroll B.J. 2006. RNA interference‐inducing hairpin RNAs in plants act through the viral defence pathway. EMBO Reports 7 (11): 1168–1175. DOI: https://doi.org/ 10.1007/s00299-010-0911-z.
41.
Gaffar F.Y. and Koch A. 2019. Catch me if you can! RNA silencing-based improvement of antiviral plant immunity. Viruses 11 (7): 673. DOI: https://doi.org/10.3390/v11070....
42.
Gan D., Zhang J., Jiang H., Jiang T., Zhu S., Cheng B. 2010. Bacterially expressed dsRNA protects maize against SCMV infection. Plant Cell Report 29 (11): 12611268. DOI: https://doi.org/10.1007/s00299....
43.
Gerber M., Sarkar S. 1989. The coat protein of Tobacco mosaic virus does not play a significant role for cross‐protection. Journal of Phytopathology 124 (4): 323–331.
44.
Gomez M.A., Lin Z.D., Moll T., Chauhan R.D., Hayden L., Renninger K., Beyene G., Taylor N.J., Carrington J.C., Staskawicz B.J. 2019. Simultaneous CRISPR/Cas9‐mediated editing of cassava eIF 4E isoforms nCBP‐1 and nCBP‐2 reduces cassava brown streak disease symptom severity and incidence. Plant Biotechnology Journal 17 (2): 421–434. DOI: https://doi.org/10.1111/pbi.12....
45.
Gonsalves D. 1998. Control of papaya ringspot virus in papaya: a case study. Annual Reviewe of Phytopathology 36 (1): 415437. DOI: https://doi.org/10.1146/annure....
46.
Gonsalves D. 2006. Transgenic papaya: development, release, impact and challenges. Advance Virus Research 67: 317354. DOI: https://doi.org/10.1016/S0065-....
47.
Grumet R. 1995. Genetic engineering for crop virus resistance. HortScience 30 (3): 449–456. DOI: 10.21273/HORTSCI.30.3.449.
48.
Higuchi M., Yoshizumi T., Kuriyama T., Hara H., Akagi C., Shimada H., Matsui M. 2009. Simple construction of plant RNAi vectors using long oligonucleotides. Journal Plant Research 122 (4): 477–482. DOI: https://doi.org/10.1007/s10265...-.
49.
Hilaire J., Tindale S., Jones G., Pingarron-Cardenas G., Bačnik K., Ojo M., Frewer L.J. 2022. Risk perception associated with an emerging agri-food risk in Europe: plant viruses in agriculture. Agriculture & Food Security 11 (1): 21. DOI: https://doi.org/10.1186/s40066....
50.
Hily J.-M., Ravelonandro M., Damsteegt V., Bassett C., Petri C., Liu Z., Scorza R. 2007. Plum pox virus coat protein gene Intron-hairpin-RNA (ihpRNA) constructs provide resistance to plum pox virus in Nicotiana benthamiana and Prunus domestica. Journal of the American Society for Horticultural Science 132 (6): 850–858. DOI: https://doi.org/10.21273/JASHS....
51.
Himber C., Dunoyer P., Moissiard G., Ritzenthaler C., Voinnet O. 2003. Transitivity‐dependent and‐independent cell‐to‐cell movement of RNA silencing. EMBO Journal 22 (17): 4523–4533. DOI: https://doi.org/10.1093/emboj/....
52.
Hjort C., Cole J., Frébort I. 2021. European genome editing regulations: Threats to the European bioeconomy and unfit for purpose. EFB Bioeconomy Journal 1: 100001. DOI: https://doi.org/10.1016/j.bioe....
53.
Hogenhout S.A., van der Hoorn R.A., Terauchi R., Kamoun S. 2009. Emerging concepts in effector biology of plant-associated organisms. Molecular Plant Microbe Interaction 22 (2): 115–122. DOI: https://doi.org/10.1094/MPMI-2....
54.
Ilardi V. and Tavazza M. 2015. Biotechnological strategies and tools for Plum pox virus resistance: trans-, intra-, cis-genesis, and beyond. Front Plant Science 6: 145843. DOI: https://doi.org/10.3389/fpls.2....
55.
Jelly N.S., Schellenbaum P., Walter B., Maillot P. 2012. Transient expression of artificial microRNAs targeting Grapevine fanleaf virus and evidence for RNA silencing in grapevine somatic embryos. Transgenic Research 21 (6): 1319–1327. DOI: https://doi.org/10.1007/s11248....
56.
Jeyaraj G., Alphonse V., Jayanthi P., Angelin N., Govindan G. 2023. Harnessing the potential of CRISPR/Cas system for enhancing virus resistance in plants: Targets, strategies, and challenges. Physiological and Molecular Plant Pathology 129: 102202. DOI: https://doi.org/10.1016/j.pmpp....
57.
Jinek M., East A., Cheng A., Lin S., Ma E., Doudna J. 2013. RNA-programmed genome editing in human cells. Elife 2: e00471. DOI: https://doi.org/10.7554/eLife.....
58.
Kalantidis K., Psaradakis S., Tabler M., Tsagris M. 2002. The occurrence of CMV-specific short RNAs in transgenic tobacco expressing virus-derived double-stranded RNA is indicative of resistance to the virus. Molecular Plant-Microbe Interactions 15 (8): 826–833. DOI: https://doi.org/10.1094/MPMI.2....
59.
Kaldis A., Berbati M., Melita O., Reppa C., Holeva M., Otten P., Voloudakis A. 2018. Exogenously applied dsRNA molecules deriving from the Zucchini yellow mosaic virus (ZYMV) genome move systemically and protect cucurbits against ZYMV. Molecular Plant Pathology 19 (4): 883895. DOI: https://doi.org/ 10.1111/mpp.12572.
61.
Khoshnami M., Zare B., Mardani-Mehrabad H., Rakhshandehroo F., Baghery M.A., Malboobi M.A. 2023. Assessment of co-infection with BNYVV and BSCTV on resistance against Rhizomania disease in transgenic sugar beet plants. Transgenic Research 32 (5): 475–485. DOI: https://doi.org/10.1007/BF0288....
62.
Kis A., Hamar É., Tholt G., Bán R., Havelda Z. 2019. Creating highly efficient resistance against wheat dwarf virus in barley by employing CRISPR/Cas9 system. Plant Biotecnology Journal 17 (6): 1004. DOI: https://doi.org/10.1111/pbi.13....
63.
Kis A., Tholt G., Ivanics M., Várallyay É., Jenes B., Havelda Z. 2016. Polycistronic artificial miRNA‐mediated resistance to W heat dwarf virus in barley is highly efficient at low temperature. Molecular Plant Pathology 17 (3): 427–437. DOI: https://doi.org/10.1111/mpp.12....
64.
Konakalla N.C., Kaldis A., Berbati M., Masarapu H., Voloudakis A.E. 2016. Exogenous application of double-stranded RNA molecules from TMV p126 and CP genes confers resistance against TMV in tobacco. Planta 244 (4): 961969. DOI: https://doi.org/10.1007/s00425....
65.
Konermann S., Lotfy P., Brideau N.J., Oki J., Shokhirev M.N., Hsu P.D. 2018. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell 173 (3): 665–676. https://doi.org/10.1016/j.cell....
66.
Kotowska‐Zimmer A., Pewinska M., Olejniczak M. 2021. Artificial miRNAs as therapeutic tools: Challenges and opportunities. Wiley Interdisciplinary Reviews RNA 12 (4): e1640. DOI: https://doi.org/10.1002/wrna.1....
67.
Kuroiwa K., Thenault C., Nogué F., Perrot L., Mazier M., Gallois J.-L. 2022. CRISPR-based knock-out of eIF4E2 in a cherry tomato background successfully recapitulates resistance to pepper veinal mottle virus. Plant Science 316: 111160. DOI: https://doi.org/10.1016/j.plan....
68.
Lau S., Mazumdar P., Hee T., Song A., Othman R., Harikrishna J. 2014. Crude extracts of bacterially-expressed dsRNA protect orchid plants against Cymbidium mosaic virus during transplantation from in vitro culture. The Journal of Horticultural Science and Biotechnology 89 (5): 569576. DOI: https://doi.org/10.1080/146203....
69.
Lerch B. 1987. On the inhibition of plant virus multiplication by ribavirin. Antiviral Research 7 (5): 257–270.
70.
Li F., Ding S.-W. 2006. Virus counterdefense: diverse strategies for evading the RNA-silencing immunity. Annual Reviewe in Microbiology 60: 503–531. DOI: https://doi.org/10.1146/annure....
71.
Lindbo J.A. 2012. A historical overview of RNAi in plants. Antiviral Resistance in Plants: Methods and Protocols 894: 1–16. DOI:
72.
Lindbo J.A., Dougherty W.G. 1992. Untranslatable transcripts of the tobacco etch virus coat protein gene sequence can interfere with tobacco etch virus replication in transgenic plants and protoplasts. Virology 189 (2): 725–733. DOI:
73.
López-Dolz L., Spada M., Daròs J.-A., Carbonell A. 2020. Fine-tune control of targeted RNAi efficacy by plant artificial small RNAs. Nucleic Acids Reseach 48 (11): 6234–6250. DOI: https://doi.org/10.1093/nar/gk....
74.
Lopez C., Cervera M., Fagoaga C., Moreno P., Navarro L., Flores R., Pena L. 2010. Accumulation of transgene‐derived siRNAs is not sufficient for RNAi‐mediated protection against Citrus tristeza virus in transgenic Mexican lime. Molecular Plant Pathology 11 (1): 33–41. DOI: https://doi.org/ 10.1093/nar/gkaa343.
75.
McKinney H. 1929. Mosaic diseases in the Canary Islands, West Africa and Gibraltar. Journal of Agricultral Research 39: 577–578.
76.
Mehta D., Stürchler A., Anjanappa R.B., Zaidi S.S.-e.-A., Hirsch-Hoffmann M., Gruissem W., Vanderschuren H. 2019. Linking CRISPR-Cas9 interference in cassava to the evolution of editing-resistant geminiviruses. Genome Biology 20 (1): 1–10. DOI: https://doi.org/10.1186/s13059....
77.
Melcher U., Muthukumar V., Wiley G.B., Min B.E., Palmer M.W., Verchot-Lubicz J., Ali A., Nelson R.S., Roe B.A., Thapa V. 2008. Evidence for novel viruses by analysis of nucleic acids in virus-like particle fractions from Ambrosia psilostachya. Journal Virological Methods 152 (1–2): 49–55. DOI: https://doi.org/10.1016/j.jvir....
78.
Mengistu A.A., Tenkegna T.A. 2021. The role of miRNA in plant–virus interaction: a review. Molecular Biology Reports 48 (3): 2853–2861. DOI: https://doi.org/10.1007/s11033....
79.
Mitter N., Worrall E.A., Robinson K.E., Li P., Jain R.G., Taochy C., Fletcher S.J., Carroll B.J., Lu G., Xu Z.P. 2017. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nature Plants 3 (2): 1–10. DOI: https://doi.org/10.1038/nplant....
80.
Moury B., Lebaron C., Szadkowski M., Ben Khalifa M., Girardot G., Bolou Bi B.A., Koné D., Nitiema L.W., Fakhfakh H., Gallois J.-L. 2020. Knock-out mutation of eukaryotic initiation factor 4E2 (eIF4E2) confers resistance to pepper veinal mottle virus in tomato. Virology 539: 11–17. DOI: https://doi.org/10.1016/j.viro....
81.
Mout R., Ray M., Tonga G.Y., Lee Y.-W., Tay T., Sasaki K., Rotello V.M. 2017. Efficient gene editing through direct cytosolic delivery of CRISPR/Cas9-ribonucleoprotein. ACS nano 11 (3): 2452. DOI: https://doi.org/10.1021/acsnan....
82.
Mujtaba M., Wang D., Carvalho L.B., Oliveira J.L., Espirito Santo Pereira A.D., Sharif R., Jogaiah S., Paidi M.K., Wang L., Ali Q. 2021. Nanocarrier-mediated delivery of miRNA, RNAi, and CRISPR-Cas for plant protection: current trends and future directions. ACS Agricultural Science & Technology 1 (5): 417–435. DOI: https://doi.org/10.1021/acsags....
83.
Namgial T., Kaldis A., Chakraborty S., Voloudakis A. 2019. Topical application of double-stranded RNA molecules containing sequences of Tomato leaf curl virus and Cucumber mosaic virus confers protection against the cognate viruses. Physiological and Molecular Plant Pathology 108: 101432. DOI: https://doi.org/10.1016/j.pmpp....
84.
Napoli C., Lemieux C., Jorgensen R. 1990. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2 (4): 279–289.
85.
Newell C., Rozman R., Hinchee M., Lawson E., Haley L., Sanders P., Kaniewski W., Tumer N., Horsch R., Fraley R. 1991. Agrobacterium-mediated transformation of Solanum tuberosum L. cv.‘Russet Burbank’. Plant Cell Report 10 (1): 3034. DOI: https://doi.org/10.1007/BF0023....
86.
Niu Q.-W., Lin S.-S., Reyes J.L., Chen K.-C., Wu H.-W., Yeh S.-D., Chua N.-H. 2006. Expression of artificial microRNAs in transgenic Arabidopsis thaliana confers virus resistance. Nature Biotechnology 24 (11): 1420–1428. DOI: https://doi.org/10.1038/nbt125....
87.
Noureen A., Zuhaib Khan M., Amin I., Zainab T., Ahmad N., Haider S., Mansoor S. 2022. Broad-spectrum resistance against multiple PVY-strains by CRSIPR/Cas13 system in Solanum tuberosum crop. GM Crops & Food 13 (1): 97–111. DOI: https://doi.org/10.3389/fgene.....
88.
O'Brien J., Hayder H., Zayed Y., Peng C. 2018. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Frontiers in Endocrinology 9: 402. DOI: https://doi.org/10.3389/fendo.....
89.
Oltmanns H., Frame B., Lee L.-Y., Johnson S., Li B., Wang K., Gelvin S.B. 2010. Generation of backbone-free, low transgene copy plants by launching T-DNA from the Agrobacterium chromosome. Plant Physiology 152 (3): 1158–1166. DOI: https://doi.org/10.1104/pp.109....
90.
Palukaitis P., Zaitlin M. 1984. A model to explain the “cross-protection” phenomenon shown by plant viruses and viroids. p. 420–430. In: “Plantmicrobe interactions: Molecular and genetic perspectives” (Kosuge T., Nester E.W., eds.). Macmillan Publishing Co., NY.
91.
Pares R., Martin A., Fitzell R. 1985. Virus-induced tip necrosis of passionfruit Passiflora edulis Sims. Australas. Plant Pathology 14: 76–78.
92.
Pechar G.S., Donaire L., Gosalvez B., García‐Almodovar C., Sánchez‐Pina M.A., Truniger V., and Aranda M.A. 2022. Editing melon eIF4E associates with virus resistance and male sterility. Plant Biotechnology Journal 20 (10): 2006–2022. DOI: https://doi.org/10.1111/pbi.13....
93.
Pennazio S., Roggero P., Conti M. 2001. A history of plant virology. Cross protection. New Microbiology 24 (1): 99–114.
94.
Pooggin M.M. 2013. How can plant DNA viruses evade siRNA-directed DNA methylation and silencing? International Journal of Molecular Science 14 (8): 1523315259. DOI: https://doi.org/10.3390/ijms 140815233o.
95.
Pooggin M.M. 2017. RNAi-mediated resistance to viruses: a critical assessment of methodologies. Current Opinion in Virology 26: 28–35. DOI: https://doi.org/ 10.1016/j.coviro.2017.07.010.
96.
Powell-Abel P., Nelson R.S., De B., Hoffmann N., Rogers S.G., Fraley R.T., Beachy R.N. 1986. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science 232 (4751): 738–743.
97.
Powell- Abel P., Nelson R.S., De B., Hoffmann N., Fraley R.T., Beachy R.N. 1989a. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science 232 (4751): 738–743.
98.
Powell- Abel P., Stark D., Sanders P., Beachy R.N. 1989b. Protection against tobacco mosaic virus in transgenic plants that express tobacco mosaic virus antisense RNA. Proceedings of the National Academy of Sciences 86 (18): 6949–6952.
99.
Ravelonandro M., Scorza R., Briard P. 2019. Innovative RNAi strategies and tactics to tackle plum pox virus (PPV) genome in Prunus domestica-Plum. Plants 8 (12): 565. DOI: https://doi.org/10.3390/plants....
100.
Rêgo-Machado C.M., Inoue-Nagata A.K., Nakasu E.Y. 2023. Topical application of dsRNA for plant virus control: a review. Tropical Plant Pathology 48 (1): 11–22. DOI: https://doi.org/10.3390/plants....
101.
Romano N., Macino G. 1992. Quelling: transient inactivation of gene expression in Neurospora crassa by transformation with homologous sequences. Molecular microbiology 6 (22): 3343–3353.
102.
Ruiz M.T., Voinnet O., Baulcombe D.C. 1998. Initiation and maintenance of virus-induced gene silencing. Plant Cell 10 (6): 937–946.
103.
Šafářová D., Brázda P., Navratil M. 2014. Effect of artificial dsRNA on infection of pea plants by pea seed-borne mosaic virus. Chezk Journal of Genetics and Plant Breeding 50 (2): 105–108. DOI: 10.17221/120/2013-CJGPB.
104.
Schwab R., Ossowski S., Riester M., Warthmann N., Weigel D. 2006, Highly specific gene silencing by artificial microRNAs in Arabidopsis. The Plant Cell 18 (5): 1121–1133. DOI: https://doi.org/10.1105/tpc.10....
105.
Sandhya D., Jogam P., Allini V.R., Abbagani S., Alok A. 2020. The present and potential future methods for delivering CRISPR/Cas9 components in plants. Journal of Genetic Engineering and Biotechnology 18 (1): 111. DOI: https://doi.org/10.1186/s43141....
106.
Senthil-Kumar M., Mysore K.S. 2011. Caveat of RNAi in plants: the off-target effect. RNAi and plant gene function analysis. Methods and Protocols 744: 13–25. DOI: https://doi.org/10.1007/978-1-....
107.
Sharma S. and Vakhlu J. 2021. Evolution and biology of CRISPR system: a new era tool for genome editing in plants. The Botanical Review 87 (4): 496–517. DOI: https://doi.org/10.1007/s00709....
108.
Shekhawat U.K., Ganapathi T.R., Hadapad A.B. 2012. Transgenic banana plants expressing small interfering RNAs targeted against viral replication initiation gene display high-level resistance to banana bunchy top virus infection. Journal of General Virology 93 (8): 1804–1813. DOI: https://doi.org/10.1099/vir.0.....
109.
Shen W., Yang G., Chen Y., Yan P., Tuo D., Li X., Zhou P. 2014. Resistance of non-transgenic papaya plants to papaya ringspot virus (PRSV) mediated by intron-containing hairpin dsRNAs expressed in bacteria. Acta Virologica 58 (3): 261266. DOI: https://doi.org/10.4149/av_201....
110.
Sherwood J. 1987. Demonstration of the specific involvement of coat protein in tobacco mosaic virus (TMV) cross protection using a TMV coat protein mutant. Journal of Phytopatology 118 (4): 358–362.
111.
Shimizu T., Nakazono-Nagaoka E., Akita F., Wei T., Sasaya T., Omura T., Uehara-Ichiki T. 2012. Hairpin RNA derived from the gene for Pns9, a viroplasm matrix protein of Rice gall dwarf virus, confers strong resistance to virus infection in transgenic rice plants. Jouranl of Biotechnology 157 (3): 421–427. DOI: https://doi.org/10.1016/j.jbio....
112.
Sijen T., Fleenor J., Simmer F., Thijssen K.L., Parrish S., Timmons L., Plasterk R.H., Fire A. 2001. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107 (4): 465–476. DOI: https://doi.org/10.1016/s0092-....
113.
Smith N.A., Singh S.P., Wang M.-B., Stoutjesdijk P.A., Green A.G., Waterhouse P.M. 2000. Total silencing by intron-spliced hairpin RNAs. Nature 407 (6802): 319–320. DOI: https://doi.org/10.1038/350303....
114.
Sokhandan-Bashir N., Gillings M.R., Bowyer J. W. 1997. Polymerase chain reaction detection and assessment of genetic variation in New South Wales isolates of passionfruit woodiness potyvirus. Australasian Plant Pathology 26: 155–166.
115.
Sokhandan-Bashir N., Gillings M., Bowyer J. 2012. A dual coat protein construct establishes resistance to passionfruit woodiness and Cucumber mosaic viruses. Journal of Agricultural Science and Technology 14 (5): 1105–1120. DOI: https://doi.org/20.1001.1.1680....
116.
Scorza R., Ravelonandro M., Callahan A.M., Cordts J.M., Fuchs M., Dunez J., Gonsalves D. 1994. Transgenic plums (Prunus domestica L.) express the plum pox virus coat protein gene. Plant Cell Reports 14 (1): 1822. DOI: https://doi.org/10.1007/BF0023....
117.
Sun L., Lin C., Du J., Song Y., Jiang M., Liu H., Zhou S., Wen F., Zhu C. 2016. Dimeric artificial microRNAs mediate high resistance to RSV and RBSDV in transgenic rice plants. Plant Cell, Tissue and Organ Culture (PCTOC) ORG 126: 127–139. DOI: https://doi.org/10.1007/s11240....
118.
Syller J. 2020. Interspecific and intraspecific interactions among plant viruses in mixed infections. Applied Plant Virology: 437–453. Elsevier. DOI: https://doi.org/10.1016/B978-0....
119.
Tabein S., Jansen M., Noris E., Vaira A.M., Marian D., Behjatnia S., Accotto G.P., Miozzi L. 2020. The induction of an effective dsRNA-mediated resistance against tomato spotted wilt virus by exogenous application of double-stranded RNA largely depends on the selection of the viral RNA target region. Frontier in Plant Science 11: 533338. DOI: https://doi.org/10.3389/fpls.2....
120.
Tang W., Weidner D.A., Hu B.Y., Newton R.J., Hu X.-H. 2006. Efficient delivery of small interfering RNA to plant cells by a nanosecond pulsed laser-induced stress wave for posttranscriptional gene silencing. Plant Science 171 (3): 375–381. DOI: https://doi.org/10.1016/j.plan....
121.
Tashkandi M., Ali Z., Aljedaani F., Shami A., Mahfouz M.M. 2018. Engineering resistance against Tomato yellow leaf curl virus via the CRISPR/Cas9 system in tomato. Plant Signaling & Behavior 13 (10): e1525996. DOI: https://doi.org/10.1080/155923....
122.
Terns M.P. and Terns R.M. 2011. CRISPR-based adaptive immune systems. Current Opinionin Microbiology 14 (3): 321–327. DOI: https://doi.org/10.1016/j.mib.....
123.
Thomas P., Kaniewski W., Lawson E. 1997. Reduced field spread of potato leafroll virus in potatoes transformed with the potato leafroll virus coat protein gene. Plant Disease 81 (12): 14471453. DOI: https://doi.org/10.1094/PDIS.1....
124.
Tripathi J.N., Ntui V.O., Ron M., Muiruri S.K., Britt A., Tripathi L. 2019. CRISPR/Cas9 editing of endogenous banana streak virus in the B genome of Musa spp. overcomes a major challenge in banana breeding. Communications Biology 2 (1): 46. DOI: https://doi.org/10.1038/s42003....
125.
Tollefson J. 2011. Brazil cooks up transgenic bean. Nature 478 (7368): 168. DOI: https://doi.org/10.1038/478168....
126.
Tricoll D.M., Carney K.J., Russell P.F., McMaster J.R., Groff D.W., Hadden K.C., Himmel P.T., Hubbard J.P., Boeshore M.L., Quemada H.D. 1995. Field evaluation of transgenic squash containing single or multiple virus coat protein gene constructs for resistance to cucumber mosaic virus, watermelon mosaic virus 2, and zucchini yellow mosaic virus. Biotechnology 13 (12): 14581465. DOI: https://doi.org/10.1038/nbt129....
127.
Urban L., Sherwood J., Rezende J., Melcher U. 1990. Examination of mechanisms of cross protection with non-transgenic plants. Paper presented at the Recognition and response in plant-virus interactions. In: “Recongnition and Response in Plant-Virus Interaction” NATO ASI Series 41. Springer DOI: https://doi.org/10.1007/978-3-....
128.
van Houdt H., Bleys A., Depicker A. 2003. RNA target sequences promote spreading of RNA silencing. Plant Physiology 131 (1): 245–253. DOI: https://doi.org/10.1104/pp.009....
129.
van Vu, T., Choudhury N.R., Mukherjee S.K. 2013. Transgenic tomato plants expressing artificial microRNAs for silencing the pre-coat and coat proteins of a begomovirus, Tomato leaf curl New Delhi virus, show tolerance to virus infection. Virus Research 172 (1–2): 35–45. DOI: https://doi.org/ 10.1016/j.virusres.2012.12.008.
130.
Vadlamudi T., Patil B.L., Kaldis A., Gopal D.V.R.S., Mishra R., Berbati M., Voloudakis A. 2020. DsRNA-mediated protection against two isolates of Papaya ringspot virus through topical application of dsRNA in papaya. Journal of Virology Methods 275: 113750. DOI: https://doi.org/10.1016/j.jvir....
131.
Voinnet O., Pinto Y.M., Baulcombe D.C. 1999. Suppression of gene silencing: A general strategy used by diverse DNA and RNA viruses of plants. Proceedings of the National Academy of Sciences 96 (24): 14147–14152. DOI: https://doi.org/10.1073/pnas.9....
132.
Wassenegger M., Pélissier T. 1998. A model for RNA-mediated gene silencing in higher plants. Plant Molecular Biology 37 (2): 349–362. DOI: https://doi.org/10.1023/a:1005....
133.
Waterhouse P.M., Graham M.W., Wang M.-B. 1998. Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proceedings of the National Academy of Sciences 95 (23): 13959–13964. DOI: https://doi.org/10.1073/pnas.9....
134.
Wei Ding S. 2000. RNA silencing. Current Opinion in Biotechnology 11 (2): 152–156. DOI: https://doi.org/10.1016/S0958-....
135.
Weinberg M.S. Morris K.V. 2016. Transcriptional gene silencing in humans. Nucleic Acids Research 44 (14): 6505–6517. DOI: 10.1093/nar/gkw139.
136.
Wieczorek P., Obrępalska-Stęplowska A. 2013. Multiplex RT-PCR reaction for simultaneous detection of Tomato torrado virus and Pepino mosaic virus co-infecting Solanum lycopersicum. Journal of Plant Protection Research 53 (3): 289–294. DOI: 10.2478/jppr-2013-0043.
137.
Wesley S.V., Helliwell C.A., Smith N.A., Wang M., Rouse D.T., Liu Q., Gooding P.S., Singh S.P., Abbott D., Stoutjesdijk P.A. 2001. Construct design for efficient, effective and high‐throughput gene silencing in plants. Plant Journal 27 (6): 581–590. DOI: https://doi.org/10.1046/j.1365....
138.
Wiedenheft B., Sternberg S.H., Doudna J.A. 2012. RNA-guided genetic silencing systems in bacteria and archaea. Nature 482 (7385): 331–338. DOI: https://doi.org/10.1038/nature....
139.
Wolt J.D., Wang K., Sashital D., Lawrence‐Dill C.J. 2016. Achieving plant CRISPR targeting that limits off‐target effects. Plant Genome 9 (3): plantgenome2016.2005.0047. DOI: https://doi.org/10.3835/plantg....
140.
Worrall E.A., Bravo-Cazar A., Nilon A.T., Fletcher S.J., Robinson K.E., Carr J.P., Mitter N. 2019. Exogenous application of RNAi-inducing double-stranded RNA inhibits aphid-mediated transmission of a plant virus. Frontier in Plant Science 10: 439135. DOI: https://doi.org/10.3389/fpls.2....
141.
Yang R., Xu H., Long M., Yu W., Lu C., Wu G., Pan N., Chen Z. 1995. Transgenic tomato plants expressing cucumber mosaic virus coat protein and their resistance to CMV. Jiangsu Journal of Agricultural Sciences/Jiangsu Nongye Xuebao 11 (1): 4044. DOI: https://doi.org/10.1023/A:1009....
142.
Ye C. and Li H. 2010. 20 Years of transgenic research in China for resistance to Papaya ringspot virus. Transgenic Plant Jounal 4: 5863. DOI: https://doi.org/10.1007/s10535....
143.
Zaidi S.S.-e.-A., Tashkandi M., Mansoor S., Mahfouz M.M. 2016. Engineering plant immunity: using CRISPR/Cas9 to generate virus resistance. Frontier in Plant Science 7: 1673. DOI: https://doi.org/10.3389/fpls.2....
144.
Zhan X., Zhang F., Zhong Z., Chen R., Wang Y., Chang L., Bock R., Nie B., Zhang J. 2019. Generation of virus‐resistant potato plants by RNA genome targeting. Plant Biotecholgy Journal 17 (9): 1814–1822. DOI: https://doi.org/10.1111/pbi.13....
145.
Zhang P., Du H., Wang J., Pu Y., Yang C., Yan R., Yang H., Cheng H., Yu D. 2020. Multiplex CRISPR/Cas9‐mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant Biotechnology Journal 18 (6): 1384–1395. DOI: https://doi.org/10.1111/pbi.13....
146.
Zhang X., Li H., Zhang J., Zhang C., Gong P., Ziaf K., Xiao F., Ye Z. 2011. Expression of artificial microRNAs in tomato confers efficient and stable virus resistance in a cell-autonomous manner. Transgenic Research 20 (3): 569581. DOI: https://doi.org/10.1007/s11248...-.
147.
Zhang S., Shen J., Li D., Cheng Y. 2021. Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics 11 (2): 614. DOI: https://doi.org/10.7150/thno.4....
148.
Zhang T., Zhao Y., Ye J., Cao X., Xu C., Chen B., An H., Jiao Y., Zhang F., Yang X. 2019. Establishing CRISPR/Cas13a immune system conferring RNA virus resistance in both dicot and monocot plants. Plant Biotechnology Journal 17 (7): 1185. DOI: https://doi.org/10.1111/pbi.13....
149.
Zhang T., Zheng Q., Yi X., An H., Zhao Y., Ma S., Zhou G. 2018. Establishing RNA virus resistance in plants by harnessing CRISPR immune system. Plant Biotechnololgy Journal 16 (8): 1415–1423. DOI: https://doi.org/10.1111/pbi.12....
150.
Zhang X., Sat, S., Ye X., Dorrance A.E., Morris T.J., Clemente T.E., Qu F. 2011. Robust RNAi-based resistance to mixed infection of three viruses in soybean plants expressing separate short hairpins from a single transgene. Phytopathology 101 (11): 12641269. DOI: https://doi.org/10.1094/PHYTO-....
151.
Zhang Z.J. 2014. Artificial trans-acting small interfering RNA: a tool for plant biology study and crop improvements. Planta 239 (6): 11391146. DOI: https://doi.org/10.1007/s00425....
152.
Zhao Y., Yang X., Zhou G., Zhang T. 2020. Engineering plant virus resistance: from RNA silencing to genome editing strategies. Plant Biotechnology Journal 18 (2): 328–336. DOI: https://doi.org/10.3389/fmicb.....
153.
Zhou L., Yuan Q., Ai X., Chen J., Lu Y., Yan F. 2022. Transgenic rice plants expressing artificial miRNA targeting the rice stripe virus MP gene are highly resistant to the virus. Biology 11 (2): 332. DOI: https://doi.org/10.3390/biolog....
154.
Zhu Y.-X., Ou-Yang W.-J., Zhang Y.-F., Chen Z.-L. 1996. Transgenic sweet pepper plants from Agrobacterium mediated transformation. Plant Cell Reports 16: 7175. DOI: https://doi.org/10.1007/BF0127....
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.