ORIGINAL ARTICLE
The effects of deltamethrin (a synthetic pyrethroid insecticide), an anionic surfactant alone, and a co-formulated mixture of these substances on the honeybee (Apis mellifera) temperature preference, CO2 emission, and expression of detoxification-related genes
1
Institute of Biology, College of Natural Sciences, University of Rzeszow, Rzeszów, Poland
2
Interdisciplinary Center for Preclinical and Clinical Research, University of Rzeszow, Poland
3
Institute of Biotechnology, College of Natural Sciences, University of Rzeszow, Rzeszów, Poland
4
Interdisciplinary Centre for Computational Modelling, College of Natural Sciences, University of Rzeszow, Rzeszów, Poland
5
Department of Regulatory and Forensic Toxicology, Institute of Medical Expertises, Łódź, Poland
6
Institute of Animal Reproduction and Food Research, Polish Academy of Science, Olsztyn, Poland
7
Department of Animal Physiology and Neurobiology, Faculty of Biological and Veterinary Sciences, N. Copernicus University, Toruń, Poland
A - Research concept and design; B - Collection and/or assembly of data; C - Data analysis and interpretation; D - Writing the article; E - Critical revision of the article; F - Final approval of article
Submission date: 2024-05-06
Acceptance date: 2024-10-09
Online publication date: 2025-06-03
Corresponding author
Aleksandra Kuliga
Institute of Animal Reproduction and Food Research, Polish Academy of Science, Olsztyn, Poland
Institute of Animal Reproduction and Food Research, Polish Academy of Science, Olsztyn, Poland
Journal of Plant Protection Research 2025;65(2):187-199
HIGHLIGHTS
- Deltam and Superam alter bee's temperature preferences.
- CO2 emissions by bees change with pesticide exposure.
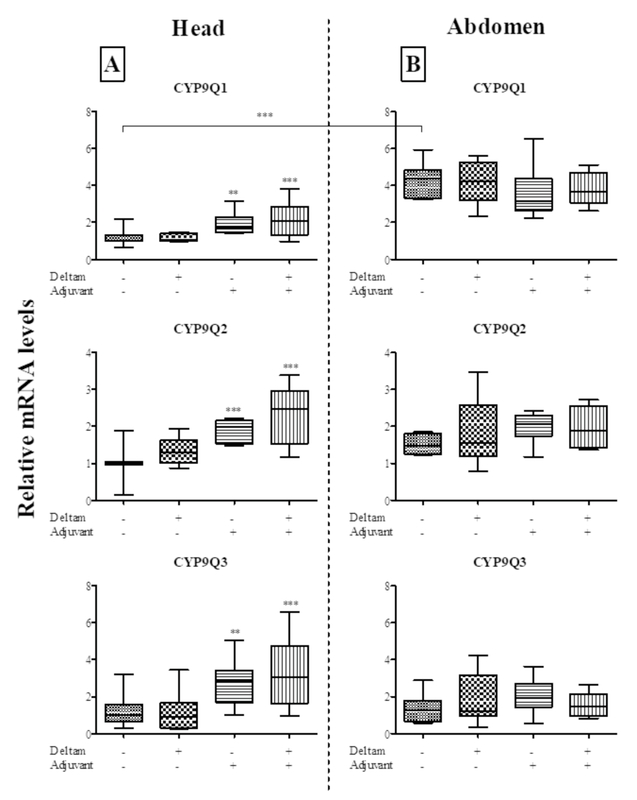
- Both substances significantly increase detox genes CYP9Q1, CYP9Q2, CYP9Q3 in bees.
- Thermal environment choice by bees is influenced by PPP formulations and adjuvants.
- Research underscores need for evaluating PPPs' impact on bee health and ecosystem.
KEYWORDS
TOPICS
ABSTRACT
Honeybees in crops are exposed to active ingredients of pesticides (plant protection
proucts – PPPs) and adjuvants used in agriculture. The aim of this research was to estimate
the effect of a sublethal dose of a pyrethroid insecticide (deltamethrin; Deltam), a plant protection
product – adjuvant (alkylbenzene sulphonic acid sodium salt; Superam 10 AL), and
a co-formulated mixture of those substances on the honeybee temperature preference, CO2
emission, and expression of detoxification-related genes (CYP9Q1, CYP9Q2, CYP9Q3).
When the measurement results were analyzed using outcome-matched statistical tests such
as the Wilcoxon test, the conclusion was reached that Deltam, Superam 10 AL and the
mixture of the two preparations significantly statistically differentiated the bees’ thermal
environment preference (a decrease in the preferred temperature by 0, 4, 0.9 and 0.5°C,
respectively), altered CO2 levels (an increase by 4.2% and a decrease by 10% and 13.5%,
respectively) and statistically significantly increased the levels of CYP9Q1, CYP9Q2 and
CYP9Q3 transcripts in the insects’ head, but not in their thorax. The results indicate that
the condition of honeybees can be affected by the finished formulation of the plant protection
product, its adjuvant, and the mixture of both acting together, with a direction of
change varying according to the studied parameters.
RESPONSIBLE EDITOR
Paweł Węgorek
CONFLICT OF INTEREST
The authors have declared that no conflict of interests exist.
REFERENCES (60)
1.
Bardach J.E., Fujiya M., Holl, A. 1965. Detergents: effects on the chemical senses of the fish Ictalurus natalis (le Sueur). Science 148 (3677): 1605–1607. DOI: 10.1126/science.148.3677.1605.
2.
Bertero A., Rivolta M., Davanzo F., Caloni F. 2020. Suspected environmental poisoning by drugs, household products and pesticides in domestic animals. Environmental Toxicology and Pharmacology 80: 103471. DOI: 10.1016/j.etap.2020.103471.
3.
Chen M., Du Y., Nomura Y., Zhorov B.S., Dong K. 2020. Chronology of sodium channel mutations associated with pyrethroid resistance in Aedes aegypti. Archives of Insect Biochemistry and Physiology 104 (2): e21686. DOI: 10.1002/arch.21686.
4.
Castañeda L.E. Figueroa C.C., Fuentes-Contreras E., Niemeyer H.M., Nespolo R.F. 2009. Energetic costs of detoxification systems in herbivores feeding on chemically defended host plants: a correlational study in the grain aphid, Sitobion avenae. Journal of Experimental Biology 212 (8): 1185–1190. DOI: 10.1242/jeb.020990.
5.
Chomczyński P., Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry 162 (1): 156–159. DOI: 10.1016/0003-2697(87)90021-2.
6.
Christiansen A., Backensfeld T., Denner K., Weitschies W. 2011. Effects of non-ionic surfactants on cytochrome P450-mediated metabolism in vitro. European Journal of Pharmaceutics and Biopharmaceutics 78 (1): 166–172. DOI: 10.1016/j.ejpb.2010.12.033.
7.
Cowan-Ellsberry C., Belanger S., Dorn P., Dyer S., McAvoy D., Sanderson H., Versteeg D., Ferrer D., Stanton K. 2014. Environmental safety of the use of major surfactant classes in North America. Critical Reviews in Environmental Science and Technology 44 (17): 1893–1993. DOI: 10.1080/10739149.2013.8037.
8.
Cserháti T., Forgács E., Oros G. 2002. Biological activity and environmental impact of anionic surfactants. Environment International 28 (5): 337–348. DOI: 10.1016/s0160-4120(02)00032-6.
9.
Ding R., Cao Z., Wang Y., Gao X., Luo H., Zhang C., Ma S., Ma X., Jin H., Lu C. 2017. The implication of p66shc in oxidative stress induced by deltamethrin. Chemico-Biological Interactions 278: 162–169. DOI: 10.1016/j.cbi.2017.10.005.
10.
Gao Y., Li X., He L., Li B., Mu W., Liu F. 2019. Role of adjuvants in the management of anthracnose – change in the crystal morphology and wetting properties of fungicides. Journal of Agricultural and Food Chemistry 67 (33): 9232–9240. DOI: 10.1021/acs.jafc.9b02147.
11.
Glitsch H.G. 2001. Electrophysiology of the sodium-potassium-ATPase in cardiac cells. Physiological Reviews 81 (4): 1791–1826. DOI: 10.1152/physrev.2001.81.4.1791.
12.
Grodzicki P., Caputa M. 2014. Diurnal and seasonal changes in thermal preference of single, isolated bees and small groups of bees (Apis mellifera L.). Journal of Insect Behavior 27 (6): 701–711. DOI: 10.1007/s10905-014-9460-6.
13.
Guedes R.N.C., Oliveira E.E., Guedes N.M.P., Ribeiro B., Serrao J.E. 2006. Cost and mitigation of insecticide resistance in the maize weevil, Sitophilus zeamais. Physiological Entomology 31 (1): 30–38. DOI: 10.1111/j.1365-3032.2005.00479.x.
14.
Guo Y., Wu H., Zhang X., Ma E., Guo Y., Zhu K. Y., Zhang J. 2016. RNA interference of cytochrome P450 CYP6F subfamily genes affects susceptibility to different insecticides in Locusta migratoria. Pest Management Science 72 (11): 2154–2165. DOI: 10.1002/ps.4248.
15.
Harrison J.W., Palmer J.H., Rittschof C.C. 2019. Altering social cue perception impacts honey bee aggression with minimal impacts on aggression-related brain gene expression. Scientific Reports 9 (1): 14642. DOI: 10.1038/s41598-019-51223-8.
16.
Hewitt A.J. 2024. Adjuvant use for the management of pesticide drift, leaching and runoff. Pest Management Science 80 (10): 4819–4827. DOI: 10.1002/ps.8255.
17.
Hodgkin A.L., Huxley A.F. 1952. A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of Physiology 117 (4): 500–554. DOI: 10.1113/jphysiol.1952.sp004764.
18.
Hołyńska-Iwan I, Szewczyk-Golec K. 2020. Pyrethroids: How they affect human and animal health? Medicina 56 (11): 582. DOI: 10.3390/medicina56110582.
19.
Hrynko I., Kaczyński P., Łozowicka B. 2021. A global study of pesticides in bees: QuEChERS as a sample preparation methodology for their analysis - Critical review and perspective. Science of the Total Environment 792: 148385. DOI: 10.1016/j.scitotenv.2021.148385.
20.
Jiang H.B., Dou W., Tang P.A., Wang J.J. 2012. Transcription and induction profiles of three novel p450 genes in Liposcelis bostrychophila (Psocoptera: Liposcelididae). Journal of Economic Entomology 105 (2): 560–572. DOI: 10.1603/ec11324.
21.
Johnson J.C. 1899. The pathology of fever. Atlanta Journal-Record of Medicine 1 (6): 433–444.
22.
Jones J.C., Helliwell P., Beekman M., Maleszka R., Oldroyd B.P. 2005. The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. Journal of Comparative Physiology A 191 (12): 1121–1129. DOI: 10.1007/s00359-005-0035-z.
23.
Kaczmarek D.K., Rzemieniecki T., Marcinkowska K., Pernak J. 2019. Synthesis, properties and adjuvant activity of docusate-based ionic liquids in pesticide formulations. Journal of Industrial and Engineering Chemistry 78: 440–447. DOI: 10.1016/j.jiec.2019.05.023.
24.
Kakko I. 2004. Toxic mechanisms of pyrethroids studied in vitro. Acta Universitatis Tamperensis, 1018.
25.
Kelly M., McBride B.W. 1990. The sodium pump and other mechanisms in large mammals. Proceedings of the Nutrition Society 49: 185–202.
26.
Kliot A., Ghanim M. 2012. Fitness costs associated with insecticide resistance. Pest Management Science 68 (11): 1431–1437. DOI: 10.1002/ps.3395.
27.
Kovac H., Stabentheiner A., Hetz S.K., Petz M., Crailsheim K. 2007. Respiration of resting honeybees. Journal of Insect Physiology 53 (12): 1250–1261. DOI: 10.1016/j.jinsphys.2007.06.019.
28.
Machani M.G., Ochomo E., Zhong D., Zhou G., Wang X., Githeko A.K., Yan G., Afrane Y.A. 2020. Phenotypic, genotypic and biochemical changes during pyrethroid resistance selection in Anopheles gambiae mosquitoes. Scientific Reports 10 (1): 19063. DOI: 10.1038/s41598-020-75865-1.
29.
Malinowski H. 1982. Wpływ temperatury na aktywność owadobójczą fotostabilnych pyretroidów. Rocznik Nauk Rolniczych 18 (1–2). (in Polish).
30.
Maliszewska J., Tęgowska E., Grajpel B., Adamkiewcz B. 2010. Effect of application of capsaicin and pyrethroid on metabolic rate in mealworm Tenebrio molitor. Ecological Chemistry and Engineering 17 (10): 1355–1359.
31.
Mao W., Schuler M.A., Berenbaum M.R. 2011. CYP9Q-mediated detoxification of acaricides in the honey bee (Apis mellifera). Proceedings of the National Academy of Sciences 108 (31): 12657–12662. DOI: 10.1073/pnas.1109535108.
32.
Medrzycki P., Sgolastra F., Bortolotti L., Bogo G., Tosi S., Padovani E., Porrini C., Sabatini A.G. 2010. Influence of brood rearing temperature on honey bee development and susceptibility to poisoning by pesticides. Journal of Apicultural Research 49 (1): 52–59. DOI: 10.3896/IBRA.1.49.1.07.
33.
Moore D., Angel J.E., Cheeseman I.M., Fahrbach S.E., Robinson G.E. 1998. Timekeeping in the honey bee colony: Integration of circadian rhythms and division of labor. Behavioral Ecology and Sociobiology 43 (3): 147–160. DOI: 10.1007/s002650050476.
34.
Moya-Quiles M.R., Muñoz-Delgado E., Vidal C.J. 1996. Effects of the pyrethroid insecticide permethrin on membrane fluidity. Chemistry and Physics of Lipids 79 (1): 21–28. DOI: 10.1016/0009-3084(95)02503-0.
35.
Natarajan G.M. 1985. Disruption of circadian rhythm of tissue respiration in Channa striatus by Metasystox®. Experientia 41 (5): 612–614. DOI: 10.1007/BF02007683.
36.
Nicolau G.Y. 1982. Circadian rhythms of RNA, DNA and protein content in the rat thyroid, adrenal and testis in chronic pesticide exposure. I. Effects of a fungicide (Mancozeb). Endocrinologie 20 (4): 249–257.
37.
Nicolau G.Y. 1983. Circadian rhythms of RNA, DNA and protein in the rat thyroid, adrenal and testis in chronic pesticide exposure. III. Effects of the insecticides (dichlorvos and trichlorphon). Physiologie 20 (2): 93–101.
38.
Piechowicz B., Gola J., Grodzicki P. 2015. Selected insecticides as modifiers of the metabolic rate in Anoplotrupes stercorosus under various thermal conditions: the effect of indoxacarb and beta-cyfluthrine. Acta Scientiarum Polonorum Silvarum Colendarum Ratio et Industria Lignaria 14 (1): 57–67. DOI: 10.17306/J.AFW.2015.1.6.
39.
Piechowicz B., Kobielska M., Koziorowska A., Podbielska M., Szpyrka E., Pieniążek M., Potocki L. 2021a. Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature. Open Chemistry 19 (1): 1242–1249. DOI: 10.1515/chem-2021-0104.
40.
Piechowicz B., Kuliga A., Kobylarz D., Koziorowska A., Zaręba L., Podbielska M., Piechowicz I., Sadło S. 2022a. A case study on the occurrence of pyrimethanil, cyprodinil and cyflufenamid residues in soil and on apple leaves, blossoms and pollen, and their transfer by worker bees to the hive. Journal of Plant Protection Research 62 (2): 176–188. DOI: 10.24425/jppr.2022.141355.
41.
Piechowicz B., Początek E., Woś I., Zaręba L., Koziorowska A., Podbielska M., Grodzicki P., Szpyrka E., Sadło S. 2022b. Insecticide and fungicide effect on thermal and olfactory behavior of bees and their disappearance in bees' tissues. Environmental Toxicology and Pharmacology 95: 103975. DOI: 10.1016/j.etap.2022.103975.
42.
Piechowicz B., Sudoł M., Grodzicki P., Podbielska M., Szpyrka E., Zwolak A., Potocki L. 2021b. The dynamics of pyrethroid residues and Cyp P450 gene expression in insects depend on the circadian clock. Environmental Research 194: 110701. DOI: 10.1016/j.envres.2020.110701.
43.
Ren X., Mao X., Cao L., Xue K., Si L., Qiu J., Schimmer A.D., Li G. 2009. Nonionic surfactants are strong inhibitors of cytochrome P450 3A biotransformation activity in vitro and in vivo. European Journal of Pharmaceutical Sciences 36 (4-5): 401–411. DOI: 10.1016/j.ejps.2008.11.002.
44.
Shafer T.J., Meyer D.A., Crofton K.M. 2005. Developmental neurotoxicity of pyrethroid insecticides: Critical review and future research needs. Environmental Health Perspectives 113 (2): 123–136. DOI: 10.1289/ehp.7254.
45.
Singh S., Mukherjee A., Jaiswal D.K., de Araujo Pereira D.A., Prasad R., Sharma M., Kuhad R.C., Shukla A.C., Verma J.P. 2022. Advances and future prospects of pyrethroids: Toxicity and microbial degradation. Science of The Total Environment 829: 154561. DOI: 10.1016/j.scitotenv.2022.154561.
46.
Straw E.A., Thompson L.J., Leadbeater E., Brown M.J.F. 2022. 'Inert' ingredients are understudied, potentially dangerous to bees and deserve more research attention. Proceedings. Biological Sciences 289 (1970): 20212353. DOI: 10.1098/rspb.2021.2353.
47.
Soderlund D.M. 2012. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Archives of Toxicology 86: 165–181. DOI: 10.1007/s00204-011-0726-x.
48.
Shen C.C., Shen D.S., Shentu J.L., Wang M.Z., Wan M.Y. 2015. Could humic acid relieve the biochemical toxicities and DNA damage caused by nickel and deltamethrin in earthworms (Eisenia foetida)? Environmental Science: Processes and Impacts 17 (12): 2074–2081. DOI: 10.1039/c5em00288e.
49.
Susmi T.S., RebelloS., Jisha M.S., Sherief P.M. 2010. Toxic effects of sodium dodecyl sulfate on rass carp Ctenopharyngodon idella. Fishery Technology 47 (2): 157–162.
50.
Terriere L.C. 1983. Enzyme induction, gene amplification and insect resistance to insecticides. 265–298. In: “Pesticide resistance in arthropods” (R.T. Roush, B.E. Tabashnik, eds.). Springer, Boston, USA. DOI: 10.1007/978-1-4684-4466-7_11.
51.
Tęgowska E. 2003. Insecticides and thermoregulation in insects. Pesticides 1: 47–75.
52.
Upham J., Acott P.D., O'Regan P., Sinal C.J., Crocker J.F.S., Geldenhuys L., Murphy M.G. 2007. The pesticide adjuvant, Toximul™, alters hepatic metabolism through effects on downstream targets of PPARα. Biochimica et Biophysica Acta - Molecular Basis of Disease 1772 (9): 1057–1064. DOI: 10.1016/j.bbadis.2007.06.003.
53.
Vandame R., Belzunces L.P. 1998. Joint actions of deltamethrin and azole fungicides on honey bee thermoregulation. Neuroscience Letters 251 (1): 57–60. DOI: 10.1016/s0304-3940(98)00494-7.
54.
Vlogiannitis S., Jonckheere W., Laget D., de Graaf D., Vontas J., Van Leeuwen T. 2021. Pyrethroid target-site resistance mutations in populations of the honey bee parasite Varroa destructor (Acari: Varroidae) from Flanders, Belgium. Experimental and Applied Acarology (85) 2021: 205–221. DOI: 10.1007/s10493-021-00665-9.
55.
Wagner A. 2005. Energy constraints on the evolution of gene expression. Molecular Biology and Evolution 22 (6): 1365–1374. DOI: 10.1093/molbev/msi126.
56.
Wang R.L., Staehelin C., Xia Q.Q., Su Y.J., Zeng R.S. 2015. Identification and characterization of CYP9A40 from the tobacco cutworm moth (Spodoptera litura), a cytochrome P450 gene induced by plant allelochemicals and insecticides. International Journal of Molecular Sciences 16 (9): 22606–22620. DOI: 10.3390/ijms160922606.
57.
Wernecke A., Eckert J.H., Forster R., Kurlemann N., Odemer R. 2022. Inert agricultural spray adjuvants may increase the adverse effects of selected insecticides on honey bees (Apis mellifera L.) under laboratory conditions. Journal of Plant Diseases and Protection 129: 93–105. DOI: 10.1007/s41348-021-00541-z.
58.
Yang Z., Zhang Y., Jiang Y., Zhu F., Zeng L., Wang Y., Lei X., Yao Y., Hou Y., Xu L., Xiong C., Yang X., Hu K. 2017. Transcriptional responses in the hepatopancreas of Eriocheir sinensis exposed to deltamethrin. PLoS ONE 12 (9): e0184581. DOI: 10.1371/journal.pone.0184581.
59.
Yu L., Tang W., He W., Ma X., Vasseur L., Baxter S.W., Yang G., Huang S., Song F., You M. 2015. Characterization and expression of the cytochrome P450 gene family in diamondback moth, Plutella xylostella (L.). Scientific Reports 5 (1): 8952. DOI: 10.1038/srep08952.
60.
Zhao G., Zhao S., Gao R., Wang R., Zhang T., Ding H., Li B., Lu C.D., Shen W.D., Wei Z. 2011. Transcription profiling of eight cytochrome P450s potentially involved in xenobiotic metabolism in the silkworm, Bombyx mori. Pesticide Biochemistry and Physiology 100 (3): 251–255. DOI: 10.1016/j.pestbp.2011.04.0.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.