RAPID COMMUNICATION
First detection of Xanthomonas campestris in Georgian hazelnuts: A threat to economic production
1
Integrated Plant Protection Research, LEPL Scientific Research Center of Agriculture, Tbilisi, Georgia
2
School of Science and Technology, University of Georgia, Tbilisi, Georgia
3
Department of Plants and Crops, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium
These authors had equal contribution to this work
A - Research concept and design; B - Collection and/or assembly of data; C - Data analysis and interpretation; D - Writing the article; E - Critical revision of the article; F - Final approval of article
Submission date: 2025-05-09
Acceptance date: 2025-10-22
Online publication date: 2025-12-10
Corresponding author
Iveta Megrelishvili
Integrated Plant Protection Research, LEPL Scientific Research Center of Agriculture, Tbilisi, Georgia
Integrated Plant Protection Research, LEPL Scientific Research Center of Agriculture, Tbilisi, Georgia
Journal of Plant Protection Research 2025;65(4):589-592
HIGHLIGHTS
- Bacterial blight symptoms have been observed on hazelnut cultivars in Georgia
- X. campetries was detected using ELISA, TaqMan triplex real-time PCR and sequencing
- This represents the first report of X. campestris in Georgia
KEYWORDS
TOPICS
ABSTRACT
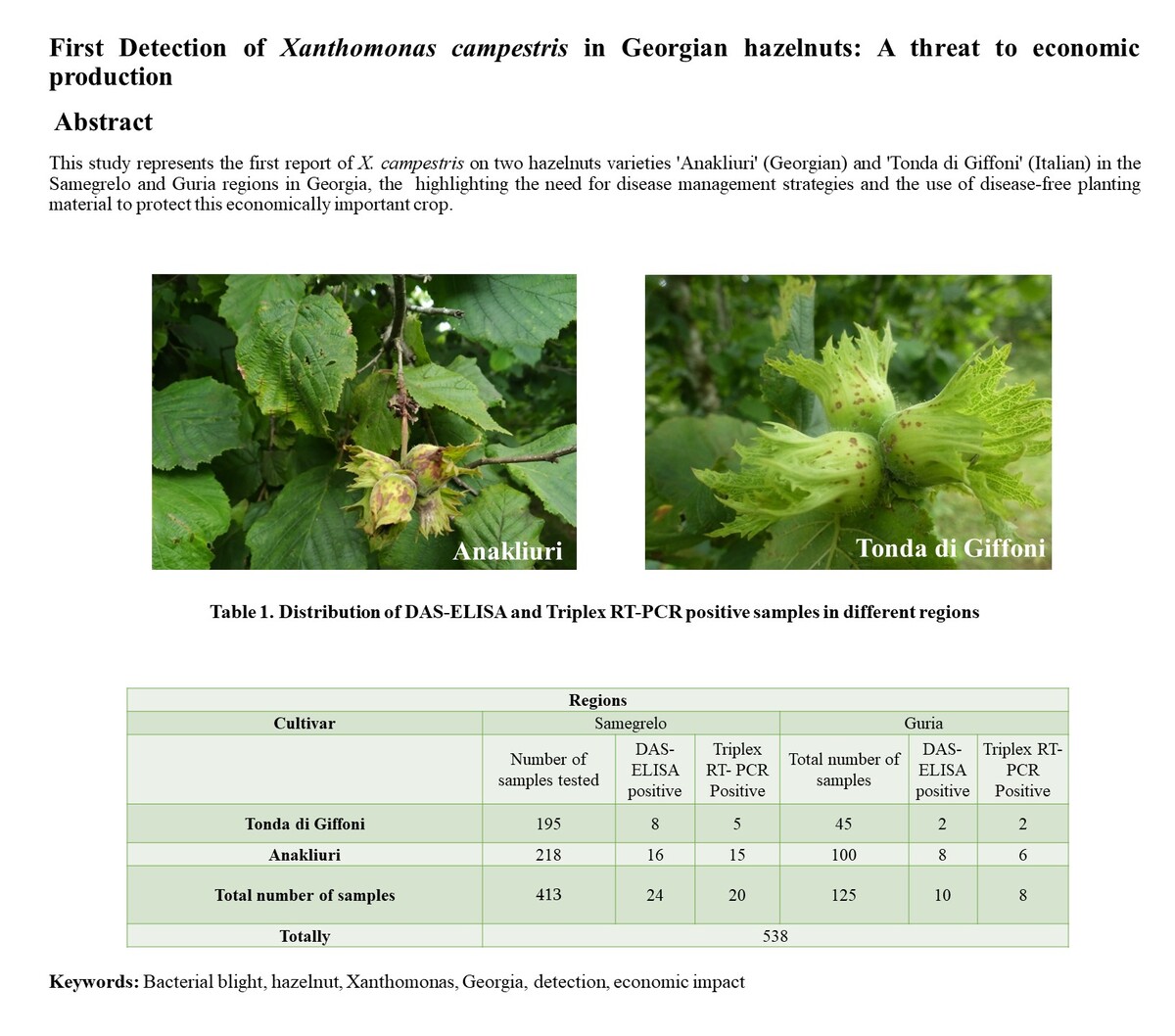
Bacterial blight, caused by Xanthomonas campestris is a major disease of hazelnut (Corylus
avellana) worldwide. Georgia is a significant hazelnut producer, but the presence of
X. campestris has not been previously confirmed. In 2022–2023, hazelnut plants in nurseries
across western Georgia were observed with symptoms of bacterial blight. Samples,
including 538 symptomatic samples of both ‘Anakliuri’ (Georgian) and ‘Tonda di Giffoni’
(Italian) hazelnut cultivars, were collected from the Samegrelo and Guria regions
in Georgia. DAS-ELISA with polyclonal antibodies identified 34 potentially positive for
X. campestris.
Additionally, samples were cultured on YPGA and King’s medium B for
presumptive X. campestris identification based on yellow colonies with subsequent confirmation
of 28 samples by a TaqMan triplex real-time PCR assay using species-specific
primers. Sanger sequencing of the 16S rRNA gene, performed on 24 of these, with further
BLAST analysis, revealed four isolates as X. campestris (GenBank Accession Numbers:
PP437082.1, PP434581.1, PP434578.1, PP434556.1) showing up to 100% nucleotide
identity to X. campestris pv. campestris strains isolated from different countries (India, Serbia,
and Mexico) and sources. Pathogenicity was confirmed by inoculating young shoots
(20–30 cm long) of 2-year-old potted hazelnut plants (‘Hall’s Giant’) with a bacterial suspension
(108 CFU · ml–1) of each isolate. Bacterial cultures were re-isolated, fulfilling Koch’s postulates.
This represents the first report of X. campestris in Georgia, highlighting the need for
disease management strategies and the use of disease-free planting material to protect this
economically important crop.
FUNDING
This work was supported by the Shota Rustaveli National
Science Foundation of Georgia (SRNSFG)
[grant number FR-22-834] and by COST action CA
21157-European Network for Innovative Woody
Plants Cloning (COPYTREE).
RESPONSIBLE EDITOR
Krzysztof Krawczyk
CONFLICT OF INTEREST
The authors have declared that no conflict of interests exist.
REFERENCES (12)
1.
Barss H.P. 1913. A new filbert disease in Oregon. Oregon Agricultural Experiment Station Biennial Crop Pest and Horticulture Report 14: 213–223.
2.
Clark M.F., Adams A.N. 1977. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. Journal of General Virology 34 (3): 475–483. DOI: https://doi.org/10.1099/0022-1....
3.
Lamichhane J.R., Fabi A., Varvaro L. 2012. Severe outbreak of bacterial blight caused by Xanthomonas arboricola pv. corylina on hazelnut, cv. Tonda di Giffoni, in central Italy. Plant Desease 96 (10): 1577. DOI: https://doi.org/10.1094/PDIS-0....
4.
Megrelishvili I., Khidesheli Z., Elbakidze T., Ujmajuridze, L., Quaglino F., Maziashvili N. 2022. Survey on phytoplasmas associated with grapevine yellows in Eastern Georgia, Caucasus region. Journal of Plant Protection Research 62 (3): 231–237. DOI: https://doi.org/10.24425/jppr.....
5.
Mikiciński A, Sobiczewski P, Berczyński S. 2012. Efficacy of fungicides and essential oils against bacterial diseases of fruit trees. Journal of Plant Protection Research 52 (4): 467-471. DOI: https://doi.org/10.2478/v10045....
6.
Miller P.W., Bollen P.W., Simmons J.E. 1949. Filbert bacteriosis and its control. Station Technical Bulletin 16: 1–107. [Online] [Available from: https://ir.library.oregonstate...] [Accessed 20 September 2025].
7.
Miller P.W., Bollen W.B., Simmons J.E., Gross H.N., Barss H.P. 1940. The pathogen of filbert bacteriosis compared with Phytomonas juglandis, the cause of walnut blight. Phytopathology 30 (9): 713–733. [Online] [Available from: CABI Digital Library] [Accessed 20 September 2025].
8.
Mori N., Quaglino F., Tessari F., Pozzebon A., Bulgari D., Casati B., Bianco P.A. 2015. Investigation on ‘bois noir’ epidemiology in north-eastern Italian vineyards through a multidisciplinary approach. Annals of Applied Biology 166 (1): 75–89. DOI: https://doi.org/10.1111/aab.12....
9.
Palacio-Bielsa A., Cubero J., Cambra M.A., Collados R., Berruete I.M., López M.M. 2011. Development of an efficient real-time quantitative PCR protocol for detection of X. arboricola pv. pruni in Prunus species. Applied and Environmental Microbiology 77 (1): 89–97. DOI: https://doi.org/10.1128/AEM.01....
10.
Pulawska J., Kaluzna M., Kolodziejska A., Sobiczewski P. 2010. Identification and characterization of X. arboricola pv. corylina causing bacterial blight of hazelnut: a new disease in Poland. Journal of Plant Pathology 92 (3): 803–806. [Online] [Available from: https://www.jstor.org/stable/4...] [Accessed 20 September 2025].
11.
Vauterin L., Hoste B., Kersters K., Swings J.1995. Reclassification of Xanthomonas. International Journal of Systematic and Evolutionary Microbiology 45 (3): 472–489. DOI: https://doi.org/10.1099/002077....
12.
Webber J.B., Putnam M., Serdani M., Pscheidt J.W., Wiman N.G., Stockwell V.O. 2020. Characterization of isolates of X. arboricola pv. corylina, the causal agent of bacterial blight, from Oregon hazelnut orchards. Journal of Plant Pathology 102 (3): 799–812. DOI: 10.1007/s42161-020-00505-6.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.